Novo Nordisk (NVO) Posts Upbeat Data From Heart Failure Study
Novo Nordisk NVO announced positive results from its late-stage study to evaluate the efficacy of Wegovy (semaglutide 2.4mg) in the treatment of patients with heart failure with preserved ejection fraction (HFpEF) and obesity. It was observed that the once-weekly dose of Wegovy caused a serious reduction in heart failure-related symptoms, overcoming physical limitations to a great extent as well as improved exercise function.
In the STEP HFpEF study, treatment with Wegovy also led to greater weight loss in adult patients with HFpEF and obesity. The safety profile of Wegovy was also consistent with that observed in previous studies of Wegovy.
The phase III STEP HFpEF has two primary endpoints, the first being the change in KCCQ-CSS, a metric for symptoms and physical limitations of HFpEF, from baseline to week 52. The other primary endpoint is a change in body weight from baseline through the same duration of the study. The study also has several key secondary endpoints, which include the change in the six-minute walking distance (6MWD) at week 52.
Year to date, shares of Novo Nordisk have shot up 36.9% compared with the industry’s 6.8% rise.
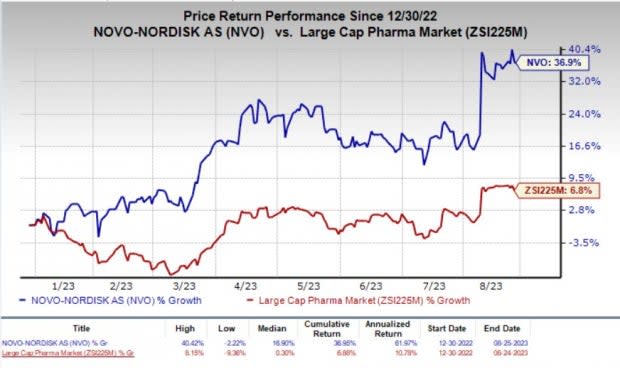
Image Source: Zacks Investment Research
Per the data readout from the study, a mean change of 16.6-point increase was observed in the KCCQ-CSS at week 52, in the treatment arm receiving Wegovy compared with 8.7 points in the placebo arm. This resulted in an estimated treatment difference of 7.8 points. A mean reduction of 13.3% in body weight was also observed upon treatment with Wegovy compared with a 2.6% reduction with placebo. A treatment difference of 10.7% weight reduction in body weight was observed.
Furthermore, treatment with Wegovy also resulted in a mean increase of 21.5m in the 6MWD at week 52 compared with only 1.2m with placebo. NVO reported that Wegovy also reduced inflammation in patients per a metric used for its measurement.
The company is also evaluating Wegovy in an ongoing STEP HFpEF-DM study to treat HFpEF and obesity in patients with type 2 diabetes. The company intends to include data from this study as well, along with that from the STEP HFpEF study, when it submits its label-expansion seeking application for Wegovy for regulatory approval. The company expects to complete the STEP HFpEF-DM study in the fourth quarter of 2024, followed by regulatory filing in the United States and EU in the first half of 2024, subject to positive outcomes.
Wegovy, a GLP-1 receptor agonist, was first approved in the United States in 2021 for weight management in people living with obesity. Currently, the drug is approved in the EU as well. It is indicated for adults as well as pediatric patients aged 12 years and older.
Novo Nordisk A/S Price and Consensus
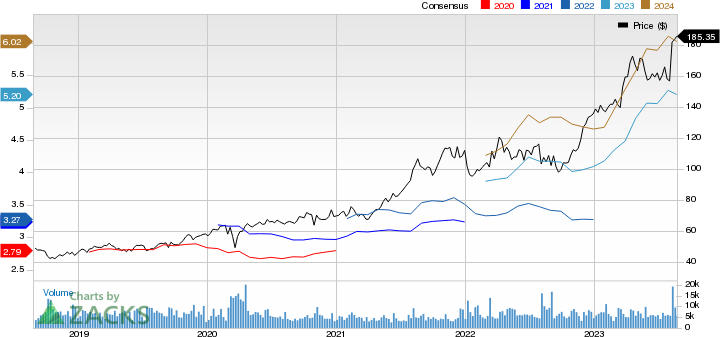
Novo Nordisk A/S price-consensus-chart | Novo Nordisk A/S Quote
Zacks Rank and Stocks to Consider
Novo Nordisk currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the pharma/biotech sector are J&J JNJ, Corcept Therapeutics CORT and Dynavax Technologies DVAX, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.73 to $10.75. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.28 to $11.30. Year to date, shares of JNJ have lost 6.6%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 62 cents to 78 cents. The estimate for Corcept’s 2024 earnings per share has also improved from 61 cents to 83 cents. Year to date, shares of CORT have climbed 56.4%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
In the past 30 days, the Zacks Consensus Estimate for Dynavax’s 2023 loss per share has narrowed from 51 cents to 24 cents. The estimate for Dynavax’s 2024 earnings per share is currently pegged at 2 cents. Year to date, shares of DVAX have risen by 39.6%.
DVAX’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 25.78%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
