Nuvalent (NUVL) Soars 36% on Upbeat Initial NSCLC Study Data
Nuvalent, Inc. NUVL, a clinical-stage company, announced positive preliminary results from the phase I dose-escalation portion of its ongoing early-mid-stage ALKOVE-1 study evaluating NVL-655 in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) and other solid tumors.
NVL-655 is Nuvalent’s novel oral brain-penetrant ALK-selective tyrosine kinase inhibitor (TKI). As of Jun 12, 2023, 57 patients (54 NSCLC and three other solid tumors patients) received 15-200 mg once daily doses of NVL-655.
Per the data readout, NVL-655 demonstrated preliminary activity in the enrolled and heavily pre-treated patient population as measured by objective response rate (ORR). Partial responses were observed in 45% of response-evaluable patients with ALK-positive NSCLC, receiving 15-150 mg once daily doses of NVL-655.
Additionally, an ORR of 65% was achieved in patients with baseline ALK resistance mutations, while an ORR of 41% was observed in patients previously treated with Pfizer’s PFE Lorbrena (lorlatinib), including cases with compound resistance mutations. Furthermore, treatment with NVL-655 showed the early indicators of central nervous system activity.
The company’s stock rallied about 35.6% on Wednesday, in response to encouraging preliminary data from the ALKOVE-1 study of NVL-655 in advanced ALK-positive NSCLC. Year to date, shares of NUVL have skyrocketed 93.1% against the industry’s 18.2% fall.
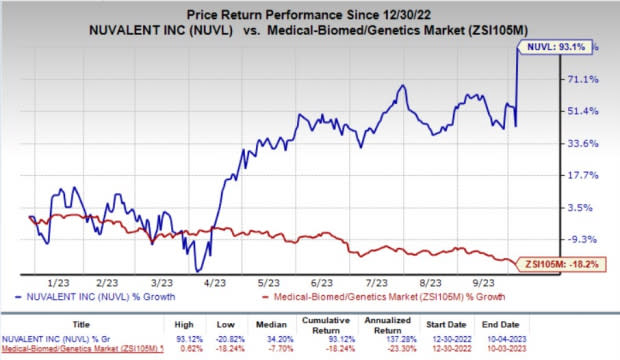
Image Source: Zacks Investment Research
The phase I dose-escalation portion of Nuvalent’s ALKOVE-1 study is currently enrolling ALK-positive NSCLC patients who have previously received at least one ALK TKI and patients with other ALK-positive solid tumors who have been previously treated with at least one prior systemic anticancer therapy.
The primary goal of the phase I portion of the study is to determine the recommended phase II dose (RP2D) and if applicable, the maximum tolerated dose (MTD) of NVL-655 in patients with ALK-positive solid tumors.
Additional objectives in the study include evaluating the safety, pharmacokinetic and tolerability profiles and the evaluation of the preliminary anti-tumor activity of NVL-655.
Nuvalent also reported that the preliminary pharmacokinetic analysis demonstrated dose-proportional exposure and preliminary pharmacodynamic analysis showed reductions, including clearance, of ALK fusion and mutation variants in ctDNA.
NVL-655 was overall well-tolerated, reporting mild treatment-related adverse events (TRAEs). Most commonly reported TRAEs included nausea, transaminase elevation, fatigue and constipation.
As of the freeze data of the preliminary analysis, an MTD was not identified and the phase I portion was still ongoing to determine the RP2D.
Pfizer’s Lorbrena is currently approved in the United States to treat first, second and third-line advanced ALK-positive metastatic NSCLC.
PFE initially received accelerated approval for Lorbrena in the second and third-line treatment of advanced ALK-positive metastatic NSCLC. It was converted to full approval along with the FDA approval of the label expansion of Lorbrena to include the first-line treatment of advanced ALK-positive metastatic NSCLC in 2021.
Pfizer’s Lorbrena is also currently approved in the EU and marketed under the brand name Lorviqua. Notably, Lorviqua is also indicated for first, second and third-line treatment of advanced ALK-positive metastatic NSCLC.
The recommended dose strength of Pfizer’s Lorbrena/Lorviqua is 100 mg orally, once daily.
Nuvalent, Inc. Price and Consensus
Nuvalent, Inc. price-consensus-chart | Nuvalent, Inc. Quote
Zacks Rank and Stocks to Consider
Currently, Nuvalent has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall medical sector are Corcept Therapeutics CORT and Better Therapeutics BTTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has remained constant at 78 cents. During the same period, the estimate for Corcept’s 2024 earnings per share has also remained constant at 83 cents. Year to date, shares of CORT have gained 27.5%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
In the past 30 days, the Zacks Consensus Estimate for Better Therapeutics’ 2023 loss per share has remained constant at 98 cents. During the same period, Better Therapeutics’ 2024 loss per share has also remained constant at 80 cents. Year to date, shares of BTTX have lost 68.7%.
BTTX’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 24.22%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Nuvalent, Inc. (NUVL) : Free Stock Analysis Report
Better Therapeutics, Inc. (BTTX) : Free Stock Analysis Report
