Olema Pharmaceuticals (OLMA) Skyrockets 471% YTD: Here's Why
Olema Pharmaceuticals OLMA is a clinical-stage biopharmaceutical company focused on developing next-generation targeted therapies for women’s cancers.
The company’s lead product candidate is palazestrant (OP-1250), a proprietary, orally available small molecule with dual activity as both a complete estrogen receptor (ER) antagonist (CERAN) and a selective ER degrader (SERD).
Currently, palazestrant is being evaluated in multiple studies, as monotherapy and in combination with other therapies, as a potential treatment for certain patients with metastatic breast cancer.
Year to date, shares of Olema have surged 471.4% against the industry’s 22.7% fall.
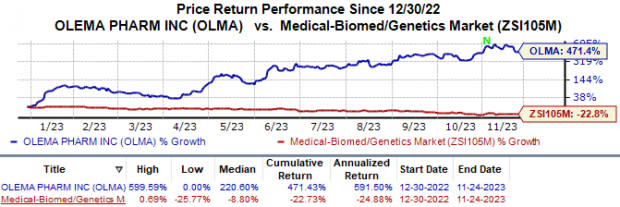
Image Source: Zacks Investment Research
This upside came after management reported encouraging data from a phase Ib/II study in May evaluating palazestrant combined with Pfizer’s PFE CDK4/6 inhibitor Ibrance (palbociclib) in patients with ER+/HER2- metastatic breast cancer.
The above results attracted interest from Wall Street, who cheered the news. Multiple analysts pointed out that the palazestrant-Ibrance combination was more effective than single-agent CDK4/6 inhibitors, which are designed to stop the growth of cancer cells.
Data from the phase Ib/II study showed that the palazestrant-Ibrance combination was safe and well tolerated, with no dose-limiting toxicities and no observed drug-drug interaction. In fact, treatment with the combination demonstrated anti-tumor activity and prolonged disease stabilization, even among those patients who were previously administered CDK4/6 inhibitors (including Pfizer’s Ibrance).
Olema intends to report updated data from the phase Ib/II study on palazestrant-Ibrance combination at the 2023 San Antonio Breast Cancer Symposium (SABCS) next month. The updated results are likely to determine whether the combination could be advanced to late-stage development.
Apart from Pfizer’s Ibrance, Olema is evaluating palazestrant in combination with Kisqali (ribociclib), another CDK4/6 inhibitor marketed by Novartis NVS, in a phase Ib/II study. Management also intends to report data from the phase Ib portion of the study at the SABCS.
The company entered into separate clinical collaboration and supply agreements with Pfizer and Novartis in 2020 for their respective CDK4/6 inhibitors. Last month, Olema expanded its collaboration with Novartis. Per the terms of the expanded partnership with Novartis, Olema will nearly double the size of the phase Ib/II study evaluating the palazestrant-Kisqali combination to around 60 patients.
Apart from the combination studies, palazestrant showed potential as a single agent in treating metastatic ER+/HER2- breast cancer. Last month, Olema reported data from a phase II study which showed that treatment with the drug achieved a median progression-free survival (PFS) of 4.6 months with a confirmed benefit rate of 40% across all 86 heavily pre-treated patients, with 42% of patients being fourth-line or later at study entry.
Based on these results, management recently started a pivotal phase III study (OPERA-01) evaluating palazestrant as monotherapy in second- and third-line metastatic breast cancer. The first patient is expected to be enrolled before this year’s end.
Currently, palazestrant is Olema’s only pipeline candidate undergoing clinical development. Apart from palazestrant, the company is planning to advance a new pipeline candidate, a novel KAT6 inhibitor, to clinical development. This new candidate, which is being developed in collaboration with Aurigene Oncology, has demonstrated the anti-tumor activity of ER+ breast cancer in preclinical studies. In this regard, management intends to submit an investigational new drug (IND) application to the FDA next year to begin clinical studies on the KAT6 inhibitor.
Olema Pharmaceuticals, Inc. Price
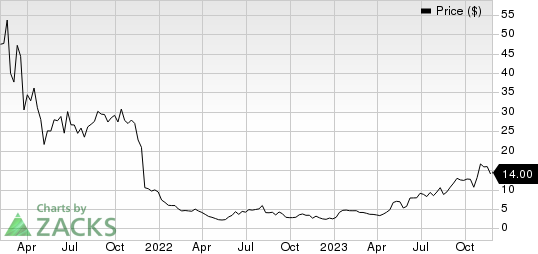
Olema Pharmaceuticals, Inc. price | Olema Pharmaceuticals, Inc. Quote
Zacks Rank & A Key Pick
Olema currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Acadia Pharmaceuticals ACAD, which carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Acadia Pharmaceuticals’ loss estimates for 2023 have narrowed from 41 cents to 33 cents per share in the past 60 days. During the same period, the estimates for 2024 earnings per share have risen from 52 cents to 94 cents. Year to date, Acadia Pharmaceuticals’ shares have gained 40.0%.
Acadia Pharmaceuticals beat earnings estimates in two of the last four quarters while missing the mark on the other two occasions, witnessing an earnings surprise of 20.69% on average. In the last reported quarter, ACAD reported an earnings surprise of 6.98%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Olema Pharmaceuticals, Inc. (OLMA) : Free Stock Analysis Report
