OPKO Health's (OPK) NGENLA Approved by FDA for GHD Treatment
OPKO Health, Inc. OPK, along with Pfizer Inc. PFE, recently confirmed that the FDA had approved NGENLA (somatrogon-ghla), a once-weekly human growth hormone analog indicated for the treatment of pediatric patients aged three years and older having growth failure due to inadequate secretion of endogenous growth hormone. NGENLA is expected to become available for U.S. prescribing in August 2023.
OPKO Health entered into a worldwide agreement with Pfizer in 2014 for the development and commercialization of somatrogon for the treatment of growth hormone deficiency (GHD). According to the agreement, OPKO Health had to conduct the clinical program while Pfizer was responsible for registering and commercializing somatrogon for GHD.
The latest approval for NGENLA in the United States follows its approval for the treatment of pediatric GHD in more than 40 markets, including Canada, Australia, Japan and EU Member States. The latest FDA approval is expected to significantly strengthen OPKO Health’s position through the global sales of NGENLA, a component of the company’s broader Pharmaceuticals business.
Background of the Approval
The FDA approval is supported by results from a multi-center, randomized, open-label, active-controlled Phase 3 study, which assessed the safety and efficacy of NGENLA when administered once-weekly compared to once-daily somatropin. The study met its primary endpoint of NGENLA’s non-inferiority compared to somatropin, as measured by annual height velocity at 12 months. NGENLA was generally well-tolerated in the study and had a safety profile comparable to somatropin.
Significance of the Approval
Per OPKO Health, GHD is a rare disease characterized by the inadequate secretion of the growth hormone somatropin from the pituitary gland. This affects approximately one in 4,000-10,000 children. If not treated, children will likely have persistent growth attenuation, a very short height in adulthood and delayed puberty. Children living with GHD may also experience challenges in relation to their physical health and mental well-being.
Per an expert familiar with the use of NGENLA, the latest regulatory approval is expected to be significant for children with GHD in the United States as it can potentially reduce the treatment burden that results from daily growth hormone injections. This will likely make NGENLA an important treatment option that can improve adherence for children being treated for GHD.
OPKO Health’s management believes that the latest FDA approval will likely better address the GHD patients and their families.
Industry Prospects
Per a report by Allied Market Research, the global human growth hormone market was valued at $3,864.00 million in 2020 and is projected to reach $9,211.63 million by 2030 at a CAGR of 9%. Factors like the development of recombinant human growth hormone drugs, a rise in pituitary dysfunction cases, and increasing cases of GHD and other growth disorders are expected to drive the market.
Given the market potential, the latest approval is expected to significantly boost OPKO Health’s global Pharmaceuticals business.
Notable Developments
In May, OPKO Health reported its first-quarter 2023 results, wherein it saw continued sales of NGENLA (somatrogon) by Pfizer in 17 countries, including Japan, Germany and the United Kingdom. OPKO Health also confirmed that Pfizer is currently expecting to launch in all priority international markets by the year’s end.
In March, OPKO Health’s ModeX Therapeutics, Inc. entered into an exclusive worldwide license and collaboration agreement with Merck to develop MDX-2201 for the Epstein-Barr virus.
Price Performance
Shares of the company have lost 32% in the past year against the industry’s 12.3% growth and the S&P 500's 15.8% rise.
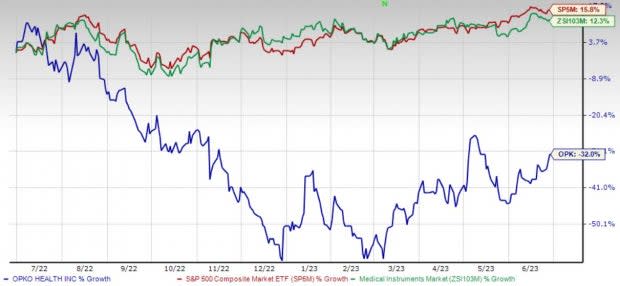
Image Source: Zacks Investment Research
Zacks Rank & Key Picks
Currently, OPKO Health carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Hologic, Inc. HOLX and HealthEquity, Inc. HQY.
Hologic, carrying a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 15.7% compared with the industry’s 12.3% growth in the past year.
HealthEquity, sporting a Zacks Rank #1 at present, has an estimated long-term growth rate of 22%. HQY’s earnings surpassed estimates in three of the trailing four quarters and missed once, the average surprise being 9.1%.
HealthEquity has gained 0.3% against the industry’s 13.9% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
OPKO Health, Inc. (OPK) : Free Stock Analysis Report
HealthEquity, Inc. (HQY) : Free Stock Analysis Report
