Panbela (PBLA) Up 11% on Update From Pancreatic Cancer Study
Panbela Therapeutics, Inc. PBLA announced that the independent Data Safety Monitoring Board (“DSMB”) of its late-stage study of ivospemin (SBP-101) in the treatment of pancreatic cancer has completed its pre-specified review of safety data for treated patients in the study.
Ivospemin, one of Panbela’s lead assets, is a polyamine metabolic inhibitor. It is currently being studied in a phase III ASPIRE study for patients with untreated metastatic pancreatic ductal adenocarcinoma. Per the DSMB’s recommendation, the study can reportedly be continued without any modifications. This recommendation was made as no safety concerns were identified upon the treatment of patients with ivospemin in the ongoing ASPIRE study.
The stock of the company surged about 11% on Monday in response to the DMSB’s positive recommendation. Year to date, shares of Panbela have plunged by 97.3% compared with the industry’s 10.6% decline.
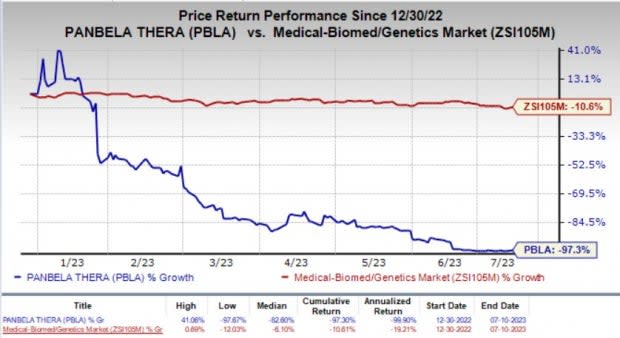
Image Source: Zacks Investment Research
The phase III ASPIRE study is evaluating ivospemin in combination with gemcitabine and nab-Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma. The current standard of care (SOC) for the treatment of patients with metastatic pancreatic ductal adenocarcinoma is the combination therapy of gemcitabine and nab-Paclitaxel.
Per the company, ivospemin has shown signs of tumor growth inhibition in studies of metastatic pancreatic cancer patients, witnessing a median overall survival of 14.6 months and an objective response rate of 48%. Both these figures exceed the results observed upon treatment with the current SOC therapy. Thus, observations suggest potential complementary activity upon treatment with ivospemin, in combination with the FDA-approved SOC chemotherapy regimen.
Additionally, data evaluated from clinical studies have shown that ivospemin treatment does not cause adverse events that are coherent with chemotherapy. Such adverse events related to chemotherapy are exacerbation of bone marrow suppression and peripheral neuropathy.
PBLA is now focused on enrolling patients in the study and completing site initiations. Interim analysis data from the study is anticipated in early 2024.
Apart from ivospemin, the company has another lead asset, flynpovi, which is being developed for the treatment of familial adenomatous polyposis (FAP). Flynpovi is a combination of CPP-1X (eflornithine) and sulindac which inhibits polyamine synthesis and increases polyamine export and catabolism.
Panbela’s third candidate, CPP-1X, is currently being developed for several indications, including prevention of gastric cancer, treatment of neuroblastoma and recent-onset type I diabetes.
Panbela Therapeutics Inc. Price and Consensus
Panbela Therapeutics Inc. price-consensus-chart | Panbela Therapeutics Inc. Quote
Zacks Rank and Other Stocks to Consider
Panbela currently has a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the same industry are ADC Therapeutics ADCT, Anixa Biosciences ANIX and Akero Therapeutics AKRO, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.58 to $2.63. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.72 to $2.49. Year-to-date, shares of ADCT have lost 38.3%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
In the past 90 days, the Zacks Consensus Estimate for Anixa Biosciences’ 2023 loss per share has narrowed from 43 cents to 33 cents. The estimate for Anixa Biosciences’ 2024 loss per share is currently pegged at 38 cents. Year-to-date, shares of ANIX have lost 29.6%.
ANIX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 31.21%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.92 to $2.80. During the same period, the estimate for AKRO’s 2024 loss per share narrowed from $3.31 to $3.27. Year-to-date, shares of AKRO have lost 17.9%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
ANIXA BIOSCIENCES INC (ANIX) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
Panbela Therapeutics Inc. (PBLA) : Free Stock Analysis Report
