Pfizer, BioNTech Seek FDA Nod for 4th COVID Shot in Older Adults
Pfizer PFE and its partner BioNTech BNTX announced that they have applied to the FDA for Emergency Use Authorization (EUA) for an additional or “fourth” booster dose of their COVID-19 vaccine for adults 65 years of age and older. The shot can be taken by any individual who has received the third booster of any of the authorized or approved COVID-19 vaccines.
The application was based on safety and efficacy data from real-world studies from Israel when the Omicron variant was widely prevalent. Data from these studies showed that in individuals who were given the fourth shot, the rates of infections were two times lower and rates of severe illness were four times lower compared to those who only received the third booster shot. In these studies, the individuals were administered the fourth booster at least four months after the third dose
Pfizer and BioNTech believe that in order for individuals to be adequately protected, additional booster doses of COVID vaccines are needed as the immunity offered by the third dose eventually fades 3 to 6 months after receiving it. The companies believe that the fourth dose can restore antibody titers to peak post-third dose titer levels and improve protection against both infection and severe disease
Pfizer and BioNTech’s booster dose or “third dose” was granted emergency approval by the FDA in December for all adults 18 years of age and older, following the completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. Later, the EUA was extended to allow its use in individuals 16 and 17 years of age and thereafter in adolescents 12 to 15 years of age.
Pfizer’s stock is down 10.9% this year so far compared with a decrease of 1% for the industry.
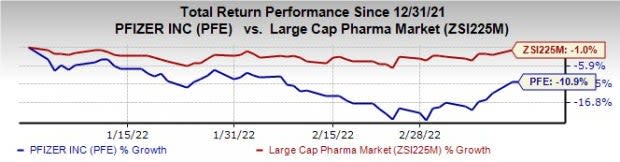
Image Source: Zacks Investment Research
Germany-based BioNTech’s shares are down 43.7% this year so far compared with the industry’s decrease of 19.7%.
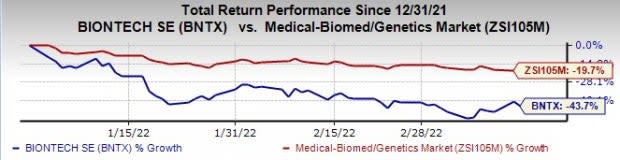
Image Source: Zacks Investment Research
Among other COVID-19 vaccine makers, Moderna’s MRNA mRNA-based COVID-19 vaccine, Spikevax is approved or authorized for use in 70 countries worldwide. Moderna delivered 807 million doses of the vaccine in 2021. A booster dose of Moderna’s vaccine has been granted EUA for use in all adults in the United States at least six months after the completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. In Europe, a booster dose can be given at least three months after the second dose for people aged 18 years and older. Meanwhile, pivotal studies are ongoing for the Omicron-specific booster candidate
A booster shot of J&J’s JNJ adenovirus-based single-shot COVID-19 vaccine has EUA for adults aged 18 and older at least two months after the primary vaccination with its vaccine or with Pfizer or Moderna’s two-shot mRNA COVID-19 vaccine regimen. J&J’s booster shot is also authorized for use in Europe.
However, Pfizer is the only company whose booster shots are authorized for use in children below the age of 18.
Both Pfizer and BioNTech have a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
