Pfizer (PFE) & BioNTech Get CHMP Nod for Updated Covid-19 Jab
Pfizer PFE and partner BioNTech BNTX announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has given a positive recommendation for its Omicron XBB.1.5-adapted monovalent Covid-19 vaccine (Comirnaty Omicron XBB.1.5).
The Comirnaty vaccines are based on BioNTech’s proprietary mRNA technology and developed and commercialized by both Pfizer and BioNTech.
Pfizer and BioNTech reported that the CHMP has recommended the EMA to approve the marketing authorization for Comirnaty Omicron XBB.1.5 as a single-dose vaccine for individuals aged five years and older, regardless of prior COVID-19 vaccination history.
The European regulatory body will review the CHMP’s recommendation and is expected to provide a final decision soon. The companies are preparing to roll out its updated vaccine to applicable EU member states, immediately after a decision from the EMA.
Year to date, shares of Pfizer have fallen 29.9% against the industry’s 8.1% rise.
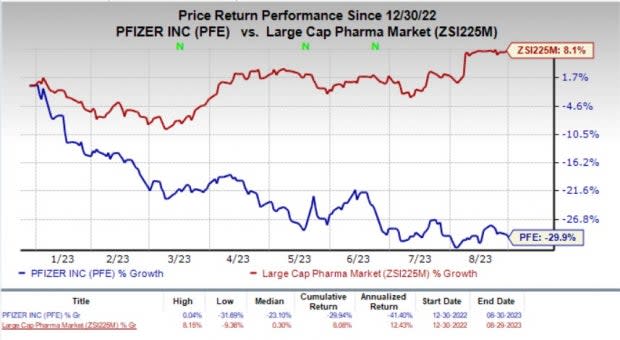
Image Source: Zacks Investment Research
Furthermore, the CHMP has also recommended the use of the updated vaccine for children aged six months to four years, as part or all of the primary three-dose vaccination series, with certain conditions. The vaccine can also be administered as a single dose for those with a history of completion of a Covid-19 primary vaccination course or prior SARS-CoV-2 infection.
The recommendation made by the CHMP was based on preclinical data which showed that the updated vaccine was effective against multiple XBB sublineages, including XBB.1.5, XBB.1.16, and XBB.2.3 compared with the Omicron BA.4/BA.5-adapted bivalent Covid-19 vaccine.
Shares of BNTX climbed 2% in the last trading session in response to the encouraging news. Year to date, BioNTech’s shares have suffered a loss of 16.7% compared with the industry’s 11.9% fall.
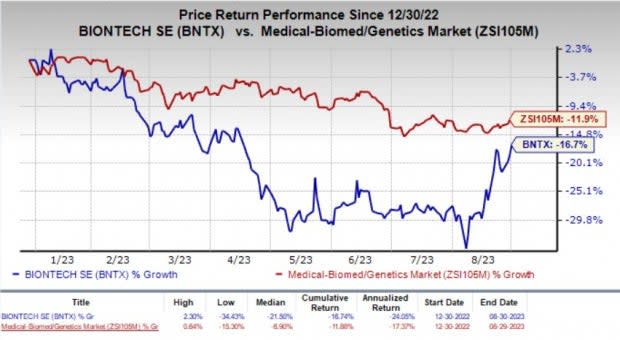
Image Source: Zacks Investment Research
The companies have been commercially manufacturing the updated Covid-19 vaccine at risk to ensure supply readiness for the upcoming fall and winter season when the demand for COVID-19 vaccination is expected to soar.
A Reuters article suggested that the Biden administration will start a fall campaign urging all Americans to inoculate themselves with the updated COVID-19 vaccines.
The report came to light amid the rising COVID-19 infection cases as new subvariants of the virus continue to emerge. Per the latest data from the Centers for Disease Control and Prevention (CDC), total hospitalizations due to COVID-19 rose by almost 19% in the most recent week.
The current updated version of the PFE/BNTX Covid-19 vaccine is also found to be effective against the EG.5.1 (also known as Eris) variant. Recently designated by the World Health Organization as a variant of interest, Eris is currently the dominant variant in the United States. Infection cases from FL.1.5.1 (also known as Fornax) are growing in the United States. Per the latest CDC data, Eris is responsible for about 21% of COVID-19 infections in the country, while Fornax accounted for more than 13% of all cases nationwide.
Pfizer and BioNTech have also filed for regulatory approval in the United States for its Omicron XBB.1.5-adapted monovalent Covid-19 vaccine for individuals aged six months and older. A decision from the FDA is expected soon.
Pfizer Inc. Price and Consensus
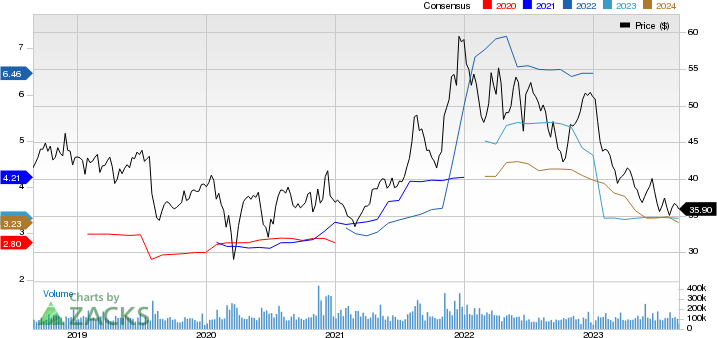
Pfizer Inc. priAce-consensus-chart | Pfizer Inc. Quote
Other players in the Covid-19 vaccine market, such as Moderna MRNA and Novavax NVAX, are currently gearing up for the anticipated increase in demand for Covid-19 vaccines in the upcoming fall and winter seasons.
Earlier this month, Moderna reported preliminary results from a clinical study showing that its updated Covid-19 vaccine mRNA-1273.815 significantly boosted neutralizing antibodies against the Eris and Fornax variants. Moderna has also submitted a regulatory application to the FDA seeking approval for mRNA-1273.815.
A final FDA decision is expected in the coming weeks for the vaccine’s launch for the upcoming fall season. A similar regulatory submission is also currently under review in Europe. Moderna remains committed to being ready with sufficient global supply during the upcoming fall season.
Last week, Novavax announced that its updated protein-based XBB Covid vaccine candidate induced neutralizing antibody against the Eris and XBB subvariant, including XBB.1.5, XBB.1.16 and XBB.2.3 variants, in non-clinical studies. In its second-quarter earnings release, NVAX reported having initiated a filing with the FDA for its updated Covid-19 vaccine. It aims to submit one in Europe in the coming weeks.
Novavax stated that it is manufacturing its protein-based monovalent XBB Covid vaccine candidate commercially, which is intended to be in the market during the upcoming fall vaccination campaign. It aims to make its updated vaccine available and accessible on par with other Covid-19 vaccines. If approved, Novavax’s Covid-19 vaccine would be the only non-mRNA protein-based XBB Covid vaccine available.
Zacks Rank
Currently, Pfizer and BioNTech carry a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
