Pharma Stock Roundup: AZN's New Acquisition, FDA Updates for NVO & LLY and More
This week, AstraZeneca AZN announced a new acquisition that will strengthen its late-stage rare disease pipeline. The FDA said it would delay its decision on Eli Lilly’s LLY Alzheimer’s disease candidate, donanemab and approved Novo Nordisk’s NVO popular obesity treatment, Wegovy, for reducing cardiovascular risks. Johnson & Johnson JNJ filed an application seeking approval for its immunology medicine, Tremfya, for ulcerative colitis (UC). Pfizer’s PFE Prevenar 20 pneumococcal conjugate vaccine was approved for use in infants and kids in the European Union.
Recap of the Week’s Most Important Stories
AstraZeneca to Buy Private Rare Disease Drugmaker: AstraZeneca announced a definitive agreement to acquire Amolyt Pharma, a private biotech making novel treatments for rare endocrine diseases, for a total consideration of up to $1.05 billion. The purchase consideration includes a $800 million upfront payment on deal closing plus a regulatory contingent milestone payment of $250 million.
The acquisition will strengthen AstraZeneca’s late-stage rare disease pipeline by adding eneboparatide (AZP-3601), which is in phase III development for chronic hypoparathyroidism. In phase II studies, eneboparatide has demonstrated the potential to improve outcomes for hypoparathyroidism patients, which can shift the treatment paradigm for the disease.
FDA to Convene Advisory Panel Meeting to Review Lilly’s Donanemab Data: The FDA is delaying its decision on Lilly’s biologics license application (BLA) for donanemab, its anti-amyloid beta antibody for early symptomatic Alzheimer's disease. Donanemab’s BLA was based on data from the TRAILBLAZER-ALZ 2 confirmatory phase III study. Lilly said an FDA advisory committee will be convened to discuss data from the TRAILBLAZER-ALZ 2 study to get more information about donanemab’s safety and effectiveness.
A date for the meeting of the Peripheral and Central Nervous System Drugs Advisory Committee has not been set yet. The FDA’s decision on donanemab was expected in the first quarter of 2024, which has now been postponed.
FDA Approves Novo Nordisk’s Wegovy for Cardiovascular Indication: The FDA approved Novo Nordisk’s blockbuster obesity injection, Wegovy, for cardiovascular indication. Wegovy is now approved to reduce the risks of major adverse cardiovascular events, including cardiovascular death, non-fatal heart attack or stroke in adults who are either overweight or obese and for established cardiovascular disease.
The approval of Wegovy for the cardiovascular indication was based on positive results from the company’s SELECT cardiovascular outcomes study, which showed that treatment with the drug, in adjunction to standard-of-care therapy, reduced the risk of MACE by 20% compared with placebo, with statistical significance.
Despite supply challenges, Wegovy is seeing strong prescription trends and is generating impressive revenues and profits for Novo Nordisk.
J&J Files Tremfya sBLA for Ulcerative Colitis Indication: J&J submitted a supplemental biologics license application (sBLA) seeking approval of Tremfya for a new indication— moderately to severely active UC.
Tremfya is presently approved to treat certain patients with plaque psoriasis and active psoriatic arthritis in several countries, including the United States and the EU. The sBLA for the UC indication was based on data from the phase III QUASAR study. Data from this study showed that a significantly greater percentage of patients with moderately to severely active UC treated with Tremfya achieved clinical remission at week 44 compared with placebo.
European Commission Approves Pfizer’s Prevenar 20 for Infants/Kids: The European Commission granted marketing approval to Pfizer’s Prevenar 20 pneumococcal conjugate vaccine for use in infants and children aged six weeks through 17 years of age.
The approval for Prevenar 20 is for the prevention of invasive disease, pneumonia and acute otitis media caused by Streptococcus pneumoniae in infants six weeks through 18 years of age. In the United States, the FDA had approved Prevnar 20 for use in infants and children aged six weeks through 17 years of age in April last year.
Pfizer announced positive overall survival data from a phase III study evaluating Adcetris in combination with lenalidomide and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) irrespective of CD30 expression.
The ECHELON-3 study demonstrated a notable improvement in the primary endpoint of overall survival compared to the standard lenalidomide and rituximab plus placebo regimen. Moreover, secondary endpoints such as progression-free survival and overall response rate also exhibited promising results, irrespective of CD30 expression. Pfizer plans to engage with the FDA for regulatory submission for the DLBCL indication based on ECHELON-3 data.
Adcetris is presently approved for seven indications for different types of lymphoma. If approved by the FDA, DLBCL will be the eighth approved indication for Adcetris.
The NYSE ARCA Pharmaceutical Index declined 0.3% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
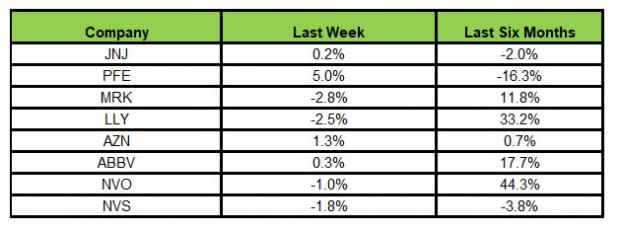
Image Source: Zacks Investment Research
In the last five trading sessions, Pfizer rose the most (5%), while Merck declined the most (2.8%).
In the past six months, Novo Nordisk has risen the most (44.3%), while Pfizer has declined the most (16.3%).
(See the last pharma stock roundup here: NVO’s New Obesity Pill Data, RHHBY, AZN’s Pipeline Updates)
What's Next in the Pharma World?
Watch for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
