Pharma Stock Roundup: FDA Priority Tag to SNY, ABBV Filings, CHMP Nod for AZN, JNJ, MRK
This week, the FDA granted priority review status to supplemental biologics license applications (sBLA) seeking approvals for the expanded use of Sanofi’s SNY blockbuster immunology medicine, Dupixent, and AbbVie’s ABBV new lymphoma drug, Epkinly. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) gave positive opinions recommending approvals of new drugs or expanded use of marketed drugs of AstraZeneca AZN, J&J JNJ and Merck MRK.
Recap of the Week’s Most Important Stories
FDA Grants Priority Tag to Sanofi’s Dupixent for COPD Indication: The FDA accepted and granted priority review to a sBLA seeking approval for Sanofi/Regeneron’s Dupixent for treating certain adult patients with uncontrolled chronic obstructive pulmonary disease (COPD). The FDA is expected to give its decision on Jun 27, 2024. Regulatory applications for Dupixent for the COPD indication are also under review in China and the European Union.
Dupixent is now approved in several countries, including the United States and EU, for five type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis, severe asthma, moderate-to-severe atopic dermatitis, eosinophilic esophagitis and prurigo nodularis. If approved by the FDA, COPD will be the sixth indication for Dupixent. The sBLA was based on data from two studies called NOTUS and BOREAS. Both studies met their primary endpoint by showing that Dupixent significantly reduced moderate or severe acute COPD exacerbations by 30% and 34%, respectively.
FDA’s Priority Tag to AbbVie’s Epkinly for Follicular Lymphoma: AbbVie announced that the FDA has granted priority review to a sBLA seeking approval for Epkinly (epcoritamab) for relapsed or refractory (R/R) follicular lymphoma (FL) after two or more therapies.
Epkinly was approved for R/R third-line diffuse large B-cell lymphoma (DLBCL) in the United States and EU in 2023. The sBLA is based on data from the FL cohort of the phase I/II EPCORE NHL study. In November 2023, the FDA granted Breakthrough Therapy Designation to Epkinly for the R/R FL indication. Epkinly is being co-developed by AbbVie and Genmab.
AbbVie announced a global licensing agreement with French biotech OSE Immunotherapeutics to develop the latter’s novel monoclonal antibody, OSE-230, for the treatment of chronic inflammation. OSE-230 is currently in pre-clinical development. Per the terms of the deal, OSE Immunotherapeutics will get an upfront payment of $48 million from AbbVie while also being entitled to receive up to an additional $665 million in milestone payments.
CHMP Recommendations for Approvals/Label Expansions: The CHMP recommended the approval of AstraZeneca’s first-in-class, oral, Factor D inhibitor Voydeya (danicopan) for treating paroxysmal nocturnal haemoglobinuria (PNH). The recommendation is an add-on to standard of care (SOC) C5 inhibitor therapy such as Ultomiris (ravulizumab) or Soliris (eculizumab) in patients who have residual haemolytic anaemia. Voydeya was approved in Japan in January, which was the drug’s first-ever regulatory approval. Regulatory applications seeking approval for Voydeya are under review in several countries, including the United States.
The CHMP gave a positive opinion for the label expansion of J&J’s CAR-T therapy, Carvykti (cilta-cel), for treating eligible patients with relapsed and refractory multiple myeloma, as early as after the first relapse. Carvykti is currently approved in the EU under conditional marketing authorisation (CMA) for treating RRMM, after three prior lines of therapy. The CHMP also recommended converting the CMA to a standard marketing authorization.
The CHMP also recommended approving Merck’s Keytruda in combination with chemotherapy as neoadjuvant treatment, then continued as monotherapy as adjuvant treatment, for the treatment of resectable non-small cell lung cancer (NSCLC) at high risk of recurrence. The European Commission’s decision on the expanded use of Keytruda for this earlier stage of NSCLC is expected in the first half of 2024. Keytruda was approved for similar use in the United States by the FDA in October 2023.
The NYSE ARCA Pharmaceutical Index rose 0.8% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
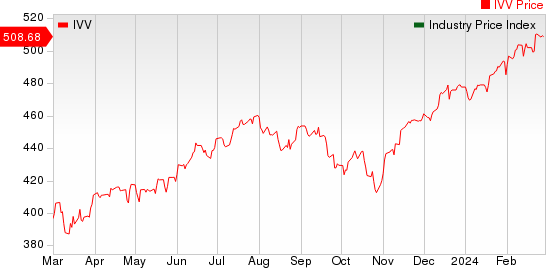
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
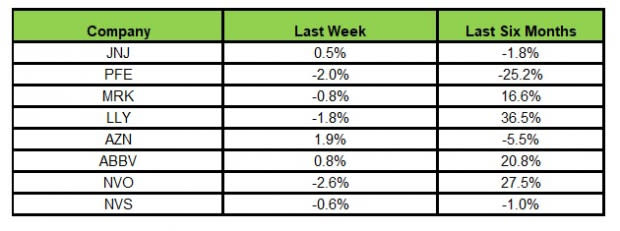
Image Source: Zacks Investment Research
In the last five trading sessions, AstraZeneca rose the most (1.9%), while Novo Nordisk declined the most (2.6%).
In the past six months, Lilly has risen the most (36.5%), while Pfizer has declined the most (25.2%).
(See the last pharma stock roundup here: Pfizer's Velsipity Receives EU Nod, ABBV Gets New CEO & More)
What's Next in the Pharma World?
Watch for regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
