Phathom Pharmaceuticals Inc (PHAT) Reports Q4 and Full Year 2023 Financials, Eyes Growth with ...
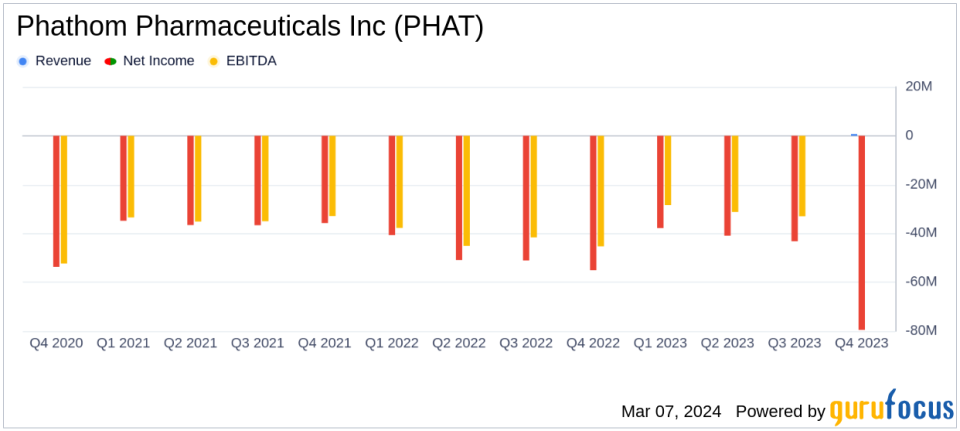
Product Revenue: PHAT reported its first product revenue of $682 thousand.
Net Loss: The company experienced a net loss of $79.6 million in Q4 and $201.6 million for the full year.
Operating Expenses: R&D expenses were $13.4 million in Q4, with a total of $49.9 million for the year.
Cash Position: PHAT ended the year with $381.4 million in cash and cash equivalents.
Non-GAAP Adjustments: Non-GAAP adjusted net loss was $45.9 million for Q4 and $129.7 million for the full year.
Stock-Based Compensation: Included in the non-GAAP adjustments, stock-based compensation expense was $24.6 million for Q4.
On March 7, 2024, Phathom Pharmaceuticals Inc (NASDAQ:PHAT) released its 8-K filing, detailing the financial results for the fourth quarter and the full year ended December 31, 2023. The company, known for its focus on developing novel treatments for gastrointestinal diseases, has recently launched VOQUEZNA, a new class of treatment for Erosive GERD, marking a significant milestone in its commercial endeavors.
Financial Performance and Business Highlights
Phathom Pharmaceuticals reported its first product revenue, netting $682 thousand, a promising start for its newly launched product, VOQUEZNA. Despite this, the company faced a substantial net loss of $79.6 million in the fourth quarter and $201.6 million for the full year. The net loss per share for the fourth quarter was $1.39, and for the full year, it was $3.93. The operating expenses for the fourth quarter were $70.4 million, with research and development expenses accounting for $13.4 million and general and administrative expenses amounting to $57 million.
Phathom ended the year with a strong cash position, with cash and cash equivalents totaling $381.4 million. This financial stability is crucial as the company continues to invest in the commercialization and development of VOQUEZNA and other pipeline products.
Non-GAAP Financial Measures and Reconciliation
The company also provided non-GAAP financial measures, which excluded non-cash share-based compensation, non-cash interest on revenue interest financing liability, and interest expense related to the amortization of debt discount. The non-GAAP adjusted net loss for the fourth quarter was $45.9 million, and for the full year, it was $129.7 million. The non-GAAP net loss per share for the fourth quarter was $0.80, and for the full year, it was $2.53.
Looking Ahead
Terrie Curran, President and CEO of Phathom, expressed optimism about the company's trajectory, citing the successful launch of VOQUEZNA and the positive reception from physicians and payers. With discussions progressing with top Pharmacy Benefit Managers (PBMs), the company anticipates prescription volume growth as formulary coverage expands. Furthermore, the potential approval of VOQUEZNA for Non-Erosive GERD in the third quarter is expected to tap into the largest segment of the GERD market, potentially driving further uptake of the product.
Phathom's strategic focus on gastrointestinal diseases and the commercialization of vonoprazan, a first-in-class potassium-competitive acid blocker, positions the company to potentially reshape the treatment landscape for GERD and other related conditions.
Conclusion
While Phathom Pharmaceuticals Inc (NASDAQ:PHAT) faces challenges with its net losses, the company's recent launch of VOQUEZNA and its solid cash position provide a foundation for future growth. The anticipated expansion into the Non-Erosive GERD market could further bolster the company's prospects. Investors and stakeholders will be watching closely as Phathom continues to navigate the commercial and regulatory landscape in the biotechnology industry.
Explore the complete 8-K earnings release (here) from Phathom Pharmaceuticals Inc for further details.
This article first appeared on GuruFocus.
