QIAGEN (QGEN) Receives FDA Approval for AYVAKIT CDX Test
QIAGEN N.V. QGEN recently announced the receipt of FDA approval for its therascreen PDGFRA RGQ PCR kit, a companion diagnostic for the gastrointestinal cancer drug Ayvakit. It aids in identifying patients with gastrointestinal stromal tumors (GIST), who may be eligible for treatment with Ayvakit, also known as avapritinib. The companion diagnostic was co-developed with Blueprint Medicines.
The latest approval adds to QIAGEN’s portfolio in precision medicine, which now includes 12 FDA-approved companion diagnostics.
More on the New Diagnostic Kit
The PDGFRA companion diagnostic assay was developed in collaboration between QIAGEN and Blueprint Medicines. The D842V somatic mutation in the PDGFRA gene is found using the real-time qualitative PCR in vitro diagnostic tool to identify whether patients may be candidates for AYVAKIT therapy.
The FDA approved a tyrosine kinase inhibitor (TKI) in 2020 to target the PDGFRA exon 18 D842V mutation. Patients with GIST who have the D842V mutation in PDGFRA exon 18 exhibit initial resistance to TKIs that have already been approved.
The therascreen PDGFRA kit utilizes genomic DNA extracted from a patient’s formalin-fixed paraffin-embedded (FFPE) tumor tissue. FFPE tumor samples are processed utilizing the Rotor-Gene Q (RGQ) MDx equipment for DNA amplification and mutation detection and the QIAamp DSP DNA FFPE Tissue Kit for sample preparation.
Significance of PDGFRA RGQ PCR Kit
Per QIAGEN’s management, therascreen's PDGFRA kit is an FDA-approved and validated test offering faster results. This makes sure that physicians get quicker results, allowing them to quickly and effectively decide on the best course of treatment for their GIST patients.
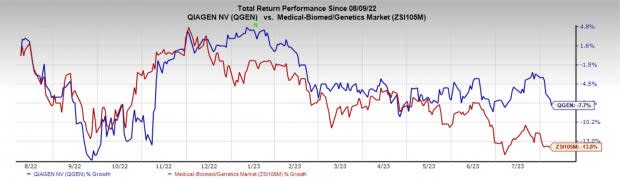
Image Source: Zacks Investment Research
The gastrointestinal tract sarcoma known as GIST is uncommon and genomically driven. A 6% of individuals with recently discovered GIST had PDGFRA exon 18 mutations, the most prevalent of which is the D842V variant. TKIs, including AYVAKIT, have significantly altered the way that GIST patients with locally progressed and metastatic diseases are treated.
Industry Prospects
Per a report by Allied Market Research, the global gastric cancer market size was valued at $2.1 billion in 2021 and is projected to reach $10.7 billion by 2031, witnessing a CAGR of 17.9% from 2022 to 2031. The increase in the occurrence of gastric cancer across the globe is projected to drive the market’s expansion.
Recent Developments
In July 2023, QIAGEN expanded its digital PCR offering for the biopharma sector's cell and gene therapy development in partnership with Niba Labs. The recent development will likely bolster QIAGEN’s Life Sciences PCR business.
The company also introduced the new CGT Viral Vector Lysis Kit, which enables a standardized workflow from cell lysates to absolute and precise quantification of viral titers for multiple serotypes.
In June 2023, QIAGEN’s ForenSeq MainstAY workflow for the U.S. National DNA Index System (“NDIS”) received FBI approval. Licensed forensic DNA laboratories will be able to process DNA casework samples and compare the resulting profiles to American databases. The NDIS CODIS database, containing 20 million offender profiles, is used by authorities across the United States to solve criminal investigations nationwide.
Price Performance
In the past year, QGEN’s shares have decreased 7.7% compared with the industry’s decline of 13.8%.
Zacks Rank and Key Picks
QIAGEN currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Penumbra, Inc. PEN, sporting a Zacks Rank #1 (Strong Buy), Integer Holdings Corporation ITGR and Intuitive Surgical, Inc. ISRG, each carrying a Zacks Rank #2 (Buy).
You can see the complete list of today’s Zacks #1 Rank stocks here.
Penumbra delivered second-quarter 2023 adjusted EPS of 43 cents, beating the Zacks Consensus Estimate by 53.6%. Revenues of $261.5 million outpaced the consensus mark by 3.3%.
Penumbra has a 2024 estimated growth rate of 57.9%. PEN’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 94.2%.
Integer Holdings reported second-quarter 2023 adjusted EPS of $1.14, beating the Zacks Consensus Estimate by 15.2%. Revenues of $400 million surpassed the Zacks Consensus Estimate by 8.9%.
Integer Holdings has a long-term estimated growth rate of 12.1%. ITGR’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 8.4%.
Intuitive Surgical reported second-quarter 2023 adjusted EPS of $1.42, beating the Zacks Consensus Estimate by 7.6%. Revenues of $1.76 billion surpassed the Zacks Consensus Estimate by 1.4%.
Intuitive Surgical has a long-term estimated growth rate of 14.5%. ISRG’s earnings surpassed estimates in three of the trailing four quarters and missed once, the average surprise being 4.2%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Intuitive Surgical, Inc. (ISRG) : Free Stock Analysis Report
QIAGEN N.V. (QGEN) : Free Stock Analysis Report
Penumbra, Inc. (PEN) : Free Stock Analysis Report
Integer Holdings Corporation (ITGR) : Free Stock Analysis Report
