QIAGEN's (QGEN) ForenSeq MainstAY Workflow Gets FBI Nod
QIAGEN N.V. QGEN recently announced that its ForenSeq MainstAY workflow for the U.S. National DNA Index System (“NDIS”) received FBI approval. Licenced forensic DNA laboratories will be able to process DNA casework samples and compare the resulting profiles to American databases.
NDIS CODIS database, containing 20 million offender profiles, are used by authorities across the United States to solve criminal investigations countrywide.
The approval builds on the NDIS approval of the MiSeq FGx Forensic Genomics System in 2019, which included the ForenSeq DNA Signature Prep Kit workflow.
The latest approval will fortify QIAGEN’s HID and Forensics business.
About ForenSeq MainstAY workflow
Launched in 2021, the ForenSeq MainstAY workflow targets 52 forensically relevant autosomal and Y-STR DNA markers that support identity confirmation and familial searching. It complies with CODIS and European standards, supports inter-database interoperability and achieves the highest discrimination of any commercially available STR kit.
The ForenSeq MainstAY workflow has been developed and commercialized by Verogen, a QIAGEN company. This system comprises the MiSeq FGx Sequencing System, the ForenSeq MainstAY Analysis Module and the high-throughput ForenSeq MainstAY kit. The workflow was submitted to NDIS for approval by the Utah Bureau of Forensic Services Laboratory System.
Significance of the Approval
The ForenSeq MainstAY workflow is a cost-effective substitute for analyzing short tandem repeats (STR) with capillary electrophoresis (CE) and facilitates volume casework for sexual assault cases. It supports high-throughput processing of up to 96 samples in one run using next-generation sequencing (NGS) technology, offers improved sensitivity leading to increased profiling efficiency, higher resolution and is extensible for applications like forensic investigative genetic genealogy (FIGG), phenotyping and additional capabilities like body fluid identification.
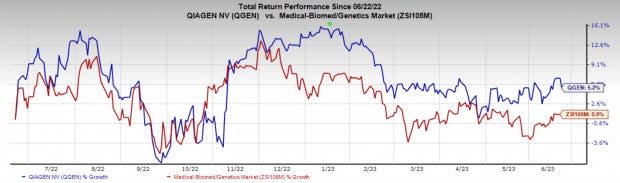
Image Source: Zacks Investment Research
This approval by the FBI reflects on the superiority of the Verogen-developed NGS-based forensic products. With ForenSeq MainstAY, QIAGEN continues to uphold its commitment to providing forensic laboratories with cost-effective NGS processes that enable them to make the switch from capillary electrophoresis without increasing their spending. The forensics community can start making this transformation thanks to NDIS compatibility, which also gives them access to federal funds for NGS validation and deployment.
Industry Prospects
Per a report by Grand View Research, the global forensic genomics market size was valued at $413.32 million in 2021 and is projected to attain a CAGR of 14.25%. The industry growth can be attributed to rapid technological advancements, increased private & government funding for the development of cost-effective forensic testing techniques and an increasing number of crimes, such as homicides, kidnapping and sexual assault.
Recent Developments
This month, QIAGEN announced that its software for interpreting and reporting variants, QIAGEN Clinical Insight (QCI Interpret), is being used in Denmark as part of a government initiative to provide sequencing-based treatments for cancer patients. The QCI Interpret solution was selected by Danish National Genome Center for variant interpretation in oncology genome sequencing.
In May 2023, QIAGEN announced that it will offer its premium enzymes as standalone solutions, giving researchers and business clients the freedom to design their own tests and workflows. QIAGEN is now a one-stop shop for all research needs, including solutions for sample preparation, automation and bioinformatics.
Price Performance
Shares of the company have gained 5.2% in a year compared with the industry’s rise of 0.9%.
Zacks Rank and Key Picks
QIAGEN carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Merit Medical Systems, Inc. MMSI and Boston Scientific Corporation BSX.
Hologic, carrying a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 20.5% compared with the industry’s 16.9% rise in the past year.
Merit Medical, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 59.5% compared with the industry’s 21.8% rise in the past year.
Boston Scientific, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11.5%. BSX’s earnings surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 1.9%.
Boston Scientific has gained 46% against the industry’s 19.8% decline in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
QIAGEN N.V. (QGEN) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
