Recce Pharmaceuticals Completes Stage 1 Data Analysis of Phase I/II Clinical Trial for the Treatment of Burn Wound Infections


Patient Wound Scores with Associated Bacterial Cultures


Patients with bacterial infections successfully treated with RECCE® 327 (R327)
Deadly bacterial pathogens treated with R327 include those identified by the World Health Organization as major threats to human health
Investigator-led clinical trial progressing to Stage 2, a ‘head-to-head’ study comparing R327 as a gel formulation (R327G) against the current standard of care in patients with burn wound infections
SYDNEY, Australia, Aug. 23, 2023 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) (the Company), the Company developing a new class of synthetic anti-infectives, is pleased to announce the Stage 1 data analysis is complete for its Phase I/II topical clinical trial of RECCE® 327 (R327) for the treatment of patients with burn wound infections.
“We thank the West Australian Government for sponsoring this investigator-led topical burn wound infection trial,” said James Graham, Chief Executive Officer of Recce Pharmaceuticals. “The patient data received has paved the way to advance this new class of anti-infective as a topical application against deadly bacterial pathogens and a broad range of infectious diseases. We look forward to launching Stage 2 as this study has facilitated.”
Clinicians have reported a visible reduction in bacterial infection within the first 24 hours of treatment, with R327 demonstrating broad-spectrum antibiotic activity against Gram-positive and Gram-negative pathogens, listed on the World Health Organization’s Priority Pathogen list of antibiotic-resistant bacteria,1 which are defined as multidrug-resistant and difficult to treat.
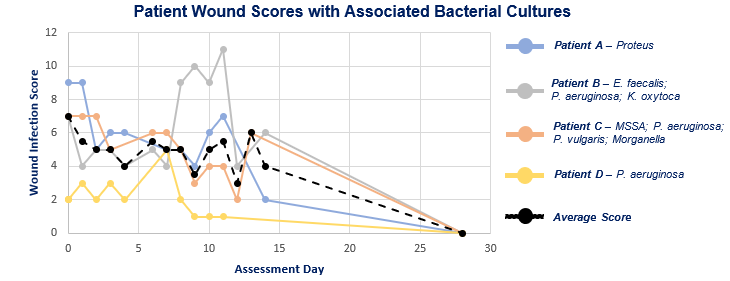
Endpoints of the trial include clinical wound assessment, graded for specific parameters such as erythema, hyper granulation, swelling, and discharge, and microbial cultures of wound swabs. Scoring the severity of a diabetic foot wound infection may help assess the severity, determine the type and urgency of antibiotic and surgical treatment needed, and predict clinical outcomes.2
All patients treated with R327 showed good indications of safety and tolerability to the compound. Observations from the study indicate that if a dose or two of R327 is missed over a period, the wound can deteriorate, a typical observation with interrupted wound treatments. Of those who did not complete dosing, one required systemic antibiotics for unrelated co-morbidities, which immediately disqualified them from continuing the study. The other, a young patient, post-wound debridement surgery, with significant, painful burns, did not respond well to gentle spray application of a cold (R327) solution to the newly raw wound surface. No serious adverse events have been reported among patients.
This West Australian Government sponsored study has led to multiple opportunities, such as the commencement of a Phase I/II trial for the treatment of diabetic foot infections, which is the largest in Australia, the use of RECCE® 327 as a gel formulation, or RECCE® 327 Gel (R327G), as a topical agent on patients under the Therapeutic Goods Administration’s (TGA) Special Access Scheme – Category A, and interest from military organizations for the use of R327 and R327G.
Recruitment has now concluded for Stage 1 of the investigator-led clinical trial due to the Fiona Stanley Hospital Burns Unit experiencing difficulty recruiting appropriate patients, primarily due to the implementation of COVID protocols resulting in preventative, systemic, antibiotic infection control practices, leading patients not to meet protocol requirements (no prior antibiotic treatment prior to enrollment), thus limiting the recruitment of the study.
In the interest of accessing a greater patient population, clinical investigators are actively preparing a new protocol in line with the progress objective of the next stage, a ‘head-to-head’ investigation. Stage 2 of the clinical trial is expected to be a randomized ‘head-to-head’ study in patients with infected burn wounds, where R327G will be compared to the current standard of care.
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) is developing a New Class of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic-resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline includes three patented, broad-spectrum, synthetic polymer anti-infectives: RECCE® 327 as an intravenous and topical therapy that is being developed for the treatment of serious and potentially life-threatening infections due to Gram-positive and Gram-negative bacteria including their superbug forms; RECCE® 435 as an orally administered therapy for bacterial infections; and RECCE® 529 for viral infections. Through their multi-layered mechanisms of action, Recce’s anti-infectives have the potential to overcome the hypercellular mutation of bacteria and viruses – the challenge of all existing antibiotics to date.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval. Further to this designation, RECCE® 327 has been included on The Pew Charitable Trusts Global New Antibiotics in Development Pipeline as the world’s only synthetic polymer and sepsis drug candidate in development. RECCE® 327 is not yet market approved for use in humans with further clinical testing required to fully evaluate safety and efficacy.
Recce wholly owns its automated manufacturing, which is supporting present clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of its technologies targeting synergistic, unmet medical needs.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 9256 2571
James.graham@recce.com.au
Media & Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media (USA)
Jordyn Temperato
LifeSci Communications
jtemperato@lifescicomms.com
Investor Relations (USA & EU)
Guillame van Renterghem
LifeSci Advisors
gvanrenterghem@lifesciadvisors.com
__________________________________
1 https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
2 https://pubmed.ncbi.nlm.nih.gov/19671126/
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/974ec61d-f38b-475c-ba34-ad7c9c145ca1

