Regeneron (REGN) Down on Ophthalmology Drug Eylea Sales Update
Shares of Regeneron Pharmaceuticals, Inc. REGN were down 7.69% after the company announced that its lead drug Eylea sales were negatively impacted by a short-term shift to off-label use of Roche’s RHHBY Avastin (bevacizumab).
On a preliminary basis, sales of Eylea in the fourth quarter came in at $1.5 billion, down 3% year over year. For 2022, sales on a preliminary basis were $6.26 billion, up 8% year over year.
Per Regeneron, sales in the fourth quarter were also impacted by the temporary closing of a fund that provides patient co-pay assistance.
Regeneron’s lead drug, Eylea, approved for various ophthalmology indications, has been a consistent performer.
We note that Roche’s Avastin is approved for the treatment of metastatic colorectal cancer (mCRC) but is used to treat age-related macular degeneration (AMD) on an off-label basis.
Regeneron is jointly developing aflibercept 8 mg with Bayer AG BAYRY. While REGN records net product sales of Eylea in the United States, Bayer records net product sales of the drug outside the United States. Regeneron records its share of profits/losses in connection with sales of Eylea outside the United States.
Regeneron had earlier announced that the two studies evaluating aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet AMD were successful. The company is evaluating a higher dose of aflibercept with less frequent injections in the targeted population. The biologics license application (BLA) was submitted in December 2022 and the company used a priority review voucher in connection with the submission. The successful label expansion will further propel sales and boost the top line.
While Eylea has maintained momentum, competition is getting stiff for the drug and a slowdown in sales will impact growth. In January 2022, Roche won FDA approval for Vabysmo (faricimab-svoa) to treat wet AMD and DME. Roche has designed Vabysmo to block pathways involving Ang-2 and VEGF-A. It has ongoing long-term extension studies for Vabysmo in people with nAMD and DME. The European Commission also approved Vabysmo (faricimab) for these indications.
Regeneron Pharmaceuticals, Inc. Price and Consensus

Regeneron Pharmaceuticals, Inc. price-consensus-chart | Regeneron Pharmaceuticals, Inc. Quote
Regeneron also announced that the Dermatologic and Ophthalmic Drugs Advisory Committee (DODAC) of the FDA met to discuss the proposed use of Eylea injection as a treatment for preterm infants with retinopathy of prematurity (ROP). The DODAC provided advice to the FDA on appropriate communication and labeling of Eylea as a treatment for ROP in preterm infants. Regeneron is in alignment with the outcome of the discussion. The DODAC’s guidance will now be considered by the FDA in its review of the supplemental biologics license application (sBLA) for EYLEA in ROP, which was previously accepted for Priority Review and assigned a target action date of Feb 11, 2023.
Regeneron’s shares have gained 9.6% in the past year against the industry’s decline of 18%.
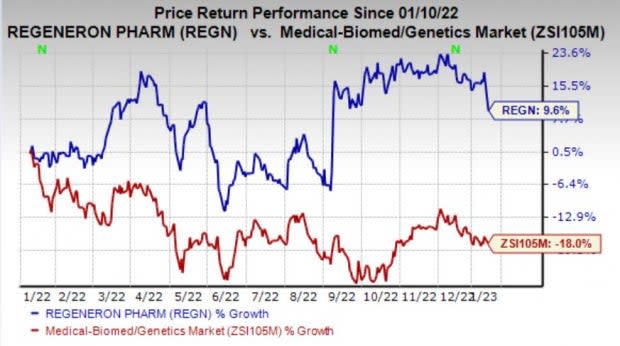
Image Source: Zacks Investment Research
Meanwhile, Regeneron also earns its share of profits/losses in connection with global sales of Dupixent. Partner Sanofi SNY records global net product sales of Dupixent. Solid sales of Dupixent (approved for use in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis) have boosted the sales of the drug.
Regeneron is also looking to diversify in the lucrative oncology space with Libtayo (cemiplimab-rwlc), indicated in certain patients with advanced basal cell carcinoma, advanced cutaneous squamous cell carcinoma and advanced non-small cell lung cancer (NSCLC). Effective Jul 1, 2022, Regeneron records global net product sales of Libtayo outside the United States and pays a royalty to Sanofi on such sales.
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
