Regeneron (REGN) Posts Positive Data on Blood Cancer Candidate
Regeneron Pharmaceuticals, Inc. REGN announced positive results from the phase I/II study on its experimental candidate, linvoseltamab.
Linvoseltamab is an investigational BCMAxCD3 bispecific antibody designed to bridge B-cell maturation antigen (“BCMA”) on multiple myeloma cells with CD3-expressing T cells to facilitate T-cell activation and cancer-cell killing.
The ongoing, open-label, multicenter phase I/II dose-escalation and dose-expansion trial is investigating linvoseltamab in patients with relapsed/refractory (“R/R”) multiple myeloma (“MM”).
MM is the second most common blood cancer.
The phase I intravenous dose-escalation portion of the trial primarily assessed safety, tolerability and dose-limiting toxicities across nine dose levels of linvoseltamab, exploring different administration regimens. The phase I of the study is complete.
The phase II dose expansion portion of the trial, LINKER-MM1, is further assessing the safety and anti-tumor activity of linvoseltamab. The primary endpoint of this study is objective response rate (“ORR”). Key secondary objectives include the duration of response, progression-free survival, rate of minimal residual disease negative status and overall survival.
Approximately 282 patients were enrolled in the study and who had received at least three prior lines of therapy or were triple refractory.
The primary endpoint analysis from the LINKER-MM1 portion showed that linvoseltamab demonstrated high rates of deep and durable responses in patients with R/R MM.
An assessment by an independent review committee demonstrated an ORR of 71% in patients treated with linvoseltamab 200 mg in the phase I/II study (n=117) after 11 months of median follow up. A complete response rate of 46% was also observed. After a minimum of 24 weeks of therapy, patients who achieved a strong partial response or better were shifted from every two weeks to every four weeks of dosing.
However, all patients treated with 200 mg experienced an adverse event (“AE”), including 85% who experienced Grade ≥3 AE.
Regeneron plans to submit these phase I/II results data to the FDA and other regulatory authorities shortly.
The candidate was granted Fast Track Designation for multiple myeloma by the FDA. Regeneron is evaluating linvoseltamab in earlier stages of the disease as well.
Shares of REGN have gained 17.7% year to date against the industry’s decline of 20.6%.
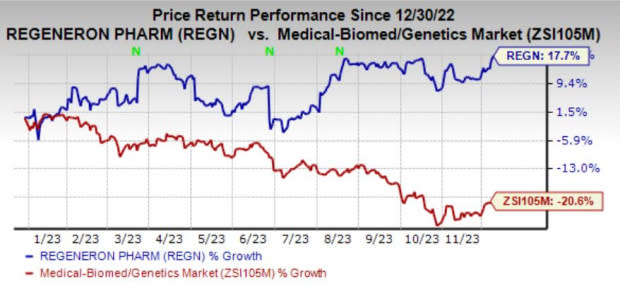
Image Source: Zacks Investment Research
Regeneron is now looking to solidify its presence in the lucrative oncology space. Its portfolio already includes an approved PD-1 inhibitor, Libtayo, approved to treat people with a type of skin cancer called cutaneous squamous cell carcinoma that has spread or cannot be cured by surgery or radiation.
The pipeline progress has been encouraging as well. In September 2023, the FDA accepted for priority review the biologics license application for odronextamab to treat adult patients with R/R follicular lymphoma and R/R diffuse large B cell lymphoma who have progressed after at least two prior systemic therapies. The regulatory body has set a target action date of Mar 31, 2024. A regulatory application for odronextamab has also been submitted to the European Union.
The FDA granted Fast Track designation to fianlimab, an antibody to LAG-3, in combination with Libtayo (cemiplimab), for the first-line treatment of patients with metastatic melanoma. A phase III study is ongoing.
The successful development of these candidates will expand Regeneron’s oncology franchise and combat the decline of its lead drug Eylea’s sales.
Eylea is approved for various ophthalmology indications. Its sales have been under pressure in 2023 due to competition from Roche’s RHHBY Vabysmo.
The uptake of Roche’s Vabysmo has been outstanding. RHHBY has designed Vabysmo to block pathways involving Ang-2 and VEGF-A. The European Commission also approved Vabysmo for these indications.
Zacks Rank and Stocks to Consider
Regeneron currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall healthcare sector are Entrada Therapeutics TRDA and Dynavax Technologies DVAX. TRDA sports a Zacks Rank #1 (Strong Buy) and DVAX carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Entrada’s loss per share estimate for 2023 has narrowed from $2.07 to 9 cents in the past 60 days. The same for 2024 has narrowed from $2.35 to $2.04 during the same time frame.
Dynavax’s loss per share estimate for 2023 has narrowed from 23 cents to 12 cents in the past 30 days. Earnings estimate for 2024 rose from 3 cents to 18 cents during the same period. Shares of DVAX have risen 26.4% year to date.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Entrada Therapeutics, Inc. (TRDA) : Free Stock Analysis Report
