Regeneron (REGN), Sanofi Get Priority Review for Dupixent sBLA
Regeneron Pharmaceuticals, Inc. REGN, along with partner Sanofi SNY, announced that the FDA has accepted the supplemental biologics license application (sBLA) seeking label expansion of blockbuster drug Dupixent (dupilumab) for yet another indication.
The sBLA is seeking approval of Dupixent for the treatment of eosinophilic esophagitis (EoE) in patients aged 12 years and above in the United States.
With the FDA granting a priority review to the sBLA, a decision from the regulatory body is expected on Aug 3, 2022.
If approved, Dupixent will become the first medicine to treat EoE in the United States.
The above-mentioned sBLA was based on data from two phase III studies that evaluated the safety and efficacy of Dupixent’s 300 mg weekly dose for treating EoE in patients aged 12 years and above, as well as data from an active long-term extension study.
Data from the same showed treatment with Dupixent significantly improved the signs and symptoms of EoE versus placebo at 24 weeks, including the ability to swallow and the reduction in eosinophil count in the esophagus.
Shares of Regeneron have rallied 10% so far this year against the industry’s decline of 10.7%.
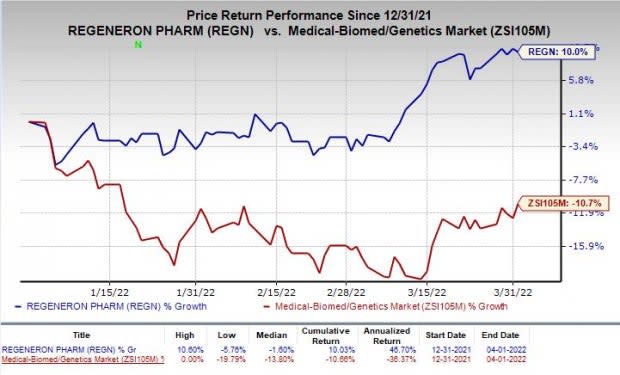
Image Source: Zacks Investment Research
The FDA has granted a Breakthrough Therapy designation to Dupixent for the treatment of patients aged 12 years and older with EoE in September 2020.
Dupixent is being jointly marketed by Regeneron and Sanofi under a global collaboration agreement. Sanofi records global net product sales of Dupixent while Regeneron records its share of profits/losses in connection with global sales of the drug.
In 2021, Dupixent generated global sales worth $6.2 billion, reflecting an increase of 53% year over year.
Dupixent is approved in the United States, the EU and some other countries for type II inflammatory diseases, namely uncontrolled chronic rhinosinusitis with nasal polyposis, moderate-to-severe asthma and moderate-to-severe atopic dermatitis. The frequent label expansion approvals are driving the drug’s sales higher.
Dupixent has become the key driver of Sanofi’s top line and profits for Regeneron. Both companies are also studying dupilumab in late-stage studies in a broad range of diseases driven by type 2 inflammations like chronic obstructive pulmonary disease, bullous pemphigoid, chronic spontaneous urticaria and some more.
Zacks Rank & Stocks to Consider
Regeneron currently carries a Zacks Rank #3 (Hold). Stocks worth considering in the biotech sector are Athersys, Inc. ATHX, and vTv Therapeutics Inc. VTVT, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Athersys’ earnings has been revised 21.7% upward for 2022 over the past 60 days.
Earnings of ATHX surpassed estimates in one of the trailing four quarters and missed the same on the other three occasions, delivering an average earnings surprise of -25.89%.
vTv Therapeutics’ loss per share estimates have narrowed 39.4% for 2022 over the past 60 days.
Earnings of VTVT surpassed estimates in two of the trailing four quarters, met the same once and missed the same on the other occasion, delivering an average surprise of 26.79%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Athersys, Inc. (ATHX) : Free Stock Analysis Report
vTv Therapeutics Inc. (VTVT) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
