Relmada (RLMD) Lead Candidate Fails Late-Stage Depression Study
Shares of Relmada Therapeutics RLMD lost 80% in one trading day on Oct 13 after management announced top-line results from the phase III RELIANCE III study. The study is evaluating its lead pipeline candidate REL-1017 as monotherapy for treating major depressive disorder (MDD).
The RELIANCE III study evaluated the REL-1017 treatment arm against the placebo arm in MDD patients. The study failed to achieve its primary of REL-1017 exhibiting statistically significant improvement in depression symptoms over placebo, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) on Day 28. While the REL-1017 treatment arm achieved a MADRS reduction of 14.8 points, the placebo arm achieved a reduction of 13.9 points.
Treatment with REL-1017 was well-tolerated in study participants and the study did not report any adverse events related to QTcF prolongation.
Relmada also reported paradoxical results in certain sites where the study was conducted. In these sites, the placebo arm outperformed the REL-1017 treatment arm. However, when these sites were excluded in a post-hoc exploratory analysis, the study showed a meaningful difference between the two treatment arms. Management is currently investigating the reasons for such differences.
Shares of Relmada Therapeutics have plunged 71.3% in the year so far compared with the industry’s 28.7% decline.
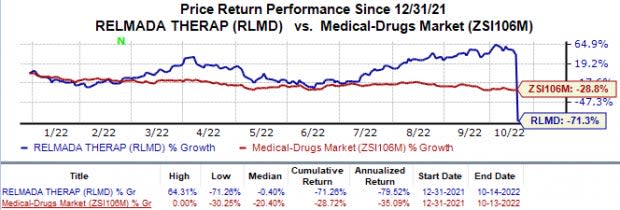
Image Source: Zacks Investment Research
A novel NMDA receptor (NMDAR), REL-1017, is designed to act as a rapid-acting oral agent for the treatment of depression and other potential indications.
REL-1017 is currently the only drug in Relmada’s pipeline undergoing late-stage development. The drug is also being evaluated in two ongoing late-stage studies, namely RELIANCE I and RELIANCE II, as a potential adjunctive treatment for MDD. These two studies are currently enrolling participants.
Another company that recently achieved success for an MDD drug in its portfolio is Axsome Therapeutics AXSM. Earlier this August, Axsome received FDA approval for its own NMDAR antagonist drug Auvelity (AXS-05), as a treatment for adults with MDD. This marks the first FDA nod to AXSM for any of its pipeline candidates.
While Relmada’s stock plunged on the news of the failure of the RELIANCE study, shares of Axsome Therapeutics gained 11.5% on the same day as the latter’s drug will likely have access to wider market following REL-1017 failure in treating MDD in the monotherapy setting. Per Axsome, following the FDA nod, Auvelity became the first and only rapid-acting oral medicine to be approved for the treatment of MDD with the labeling of statistically significant antidepressant efficacy versus placebo starting at week one.
Relmada Therapeutics, Inc. Price
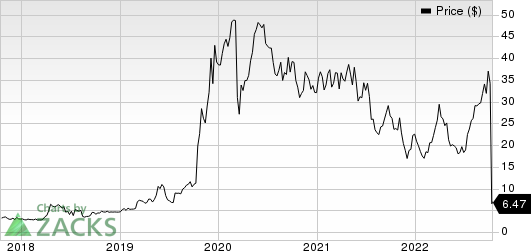
Relmada Therapeutics, Inc. price | Relmada Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Relmada Therapeutics currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stock in the overall healthcare sector includes Morphic MORF and Aerie Pharmaceuticals AERI, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Aerie Pharmaceuticals’ 2022 loss per share have narrowed from $1.83 to $1.82. During the same period, the loss estimates per share for 2023 have narrowed from $1.01 to $0.96. Shares of Aerie Pharmaceuticals have gained 116.8% in the year-to-date period.
Earnings of Aerie Pharmaceuticals beat estimates in two of the last four quarters and missed the mark twice, witnessing a surprise of 70.27% on average. In the last reported quarter, AERI delivered an earnings surprise of 38.46%.
In the past 60 days, estimates for Morphic’s 2023 loss per share have narrowed from $3.77 to $3.61. Shares of Morphic have lost 41.4% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
Axsome Therapeutics, Inc. (AXSM) : Free Stock Analysis Report
Relmada Therapeutics, Inc. (RLMD) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
