Roche's (RHHBY) Application for PNH Drug Accepted by FDA
Roche Group RHHBY member Genentech has received a significant boost following the acceptance of its biologics license application (BLA) for pipeline candidate crovalimab by the FDA.
Crovalimab, an innovative anti-C5 recycling monoclonal antibody, offers new hope for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare and life-threatening blood condition.
This development not only promises improved patient care but also serves as a strategic move to expand RHHBY's pharmaceutical portfolio.
The FDA's acceptance of the BLA was based on compelling results from the late-stage COMMODORE 2 study, which demonstrated that crovalimab effectively achieved disease control and exhibited excellent tolerability in individuals with PNH.
The phase III COMMODORE 2 study demonstrated that crovalimab, administered subcutaneously every four weeks, achieved disease control and proved non-inferior in safety compared to Soliris, a standard intravenous treatment given every two weeks.
Additionally, the consistent benefit-risk profile of crovalimab was supported by the phase III COMMODORE 1 study.
What sets crovalimab apart is its potential to become the first monthly subcutaneous treatment for PNH, allowing patients the option to self-administer outside of a supervised healthcare setting. This feature not only enhances patient convenience but also aligns with the growing trend of personalized healthcare.
PNH affects around 20,000 people worldwide and poses a severe threat to their well-being. The disease is characterized by the destruction of red blood cells by the complement system, part of the innate immune system. This leads to debilitating symptoms such as anemia, fatigue, blood clots and the potential development of kidney disease. Existing treatments for PNH involve C5 inhibitors that block the complement system cascade. Crovalimab represents a novel and promising addition to this therapeutic landscape.
What makes crovalimab particularly exciting is its unique recycling mechanism within the bloodstream. By binding to C5 and blocking the final step of the complement cascade, crovalimab offers sustained complement inhibition through low-dose subcutaneous administration every four weeks.
This approach not only improves the patient's quality of life but also has the potential to reduce treatment-related healthcare costs.
Global phase III data from both COMMODORE 1 and 2 studies have been submitted to regulatory authorities worldwide. Filing applications have been accepted in the EU, China and Japan, and submissions to other regulatory authorities around the world are ongoing.
Roche is also evaluating crovalimab in a broad clinical development program, including five ongoing phase III studies and three earlier phase studies in PNH and other complement-mediated diseases.
The potential approval of the candidate will broaden Roche’s already diversified portfolio.
We note that Soliris is marketed by AstraZeneca AZN following its acquisition of Alexion. The acquisition also added Ultomiris, as well as a growing pipeline of candidates in rare diseases, to AstraZeneca’s portfolio.
Roche’s stock has declined 9.3% in the year-to-date period against the industry’s growth of 7.2%.
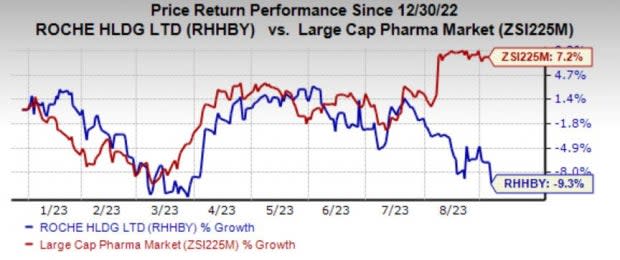
Image Source: Zacks Investment Research
Roche’s performance in the first half was decent, excluding COVID-19-related sales. New drugs, namely Ocrevus, Hemlibra, Evrysdi and Tecentriq, boosted growth. The uptake of the new eye drug Vabysmo (launched at the beginning of 2022) was outstanding.
However, sales are likely to be affected by the expected plunge in sales of COVID-19 products of nearly CHF 5 billion. Competition from biosimilars for established cancer medicines like Avastin, MabThera/Rituxan and Herceptin is also likely to hurt sales.
Zacks Rank & Stocks to Consider
Roche currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector are Dynavax Technologies DVAX and Exelixis EXEL, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss estimates for Dynavax for 2023 have narrowed to 24 cents from 56 cents in the past 60 days, while the earnings estimate for 2024 is currently pegged at 2 cents per share.
Shares of EXEL have gained 38.9% year to date. Earnings estimates for 2023 have risen by 9 cents to 98 cents.
Disclaimer: This article has been written with the assistance of Generative AI. However, the author has reviewed, revised, supplemented, and rewritten parts of this content to ensure its originality and the precision of the incorporated information.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
