Roche's (RHHBY) Xolair Gets Approval for Food Allergies
Roche RHHBY announced that the FDA has approved a label expansion of the asthma drug Xolair (omalizumab).
The regulatory body approved the drug for the reduction of allergic reactions, including anaphylaxis, that may occur with accidental exposure to one or more foods in adult and pediatric patients aged one year and older with IgE-mediated food allergy.
The FDA approval is based on positive data from the late-stage OUtMATCH study, which evaluated Xolair in patients aged between one and 55 years who are allergic to peanuts and at least two other food allergens, including milk, egg, wheat, cashew, hazelnut and walnut.
Data from the study showed a significantly higher proportion of food allergy patients as young as one year, treated with Xolair, could tolerate small amounts of peanut, milk, egg and cashew without an allergic reaction, compared to placebo.
A statistically significant higher proportion of patients treated with Xolair for 16-20 weeks tolerated at least 600 mg of peanut protein without moderate to severe allergic symptoms compared to 5% of those treated with a placebo.
Additionally, a statistically significant higher proportion of patients treated with Xolair compared to placebo tolerated at least 1,000 mg of protein from milk, egg, or cashew without moderate to severe allergic symptoms.
The OUtMATCH study is sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
Roche emphasized that although patients evaluated in the study tolerated these amounts of food, treatment with Xolair should be done with continued food allergen avoidance.
Safety findings were consistent with the known safety profile of Xolair across its additional indications.
Xolair is already approved for the treatment of moderate to severe persistent allergic asthma, chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
Xolair generated sales of CHF 2.2 billion in 2023, up 5% from the 2022 level.
Approval of a fourth indication will boost sales, given the prevalence of food allergies and lack of effective treatments. Also, the drug faces stiff competition in the asthma space from the likes of Sanofi and Regeneron’s REGN Dupixent, among others.
Approximately 3.4 million children and 13.6 million adults in the country have been diagnosed with IgE-mediated food allergies.
The recommended Xolair dosage (subcutaneous injection) for the treatment of food allergy is 75-600 mg, once every two or four weeks.
Roche has a collaboration agreement with fellow Swiss pharma company, Novartis NVS, for Xolair.
Roche and Novartis co-promote Xolair in the United States, while Novartis records all sales of Xolair outside the country.
Roche has lost 10.7% in the past six months against the industry’s growth of 12.2%.
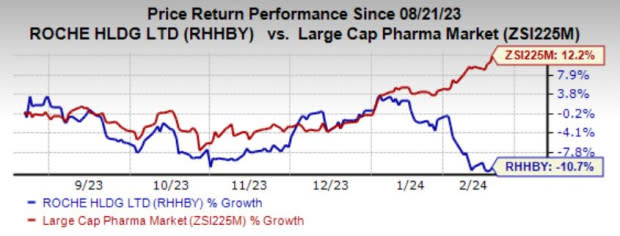
Image Source: Zacks Investment Research
Roche’s performance in 2023 was decent, excluding COVID-19-related sales. However, the outlook for 2024 is ordinary. Vabysmo, Ocrevus, Hemlibra and Polivy boosted its growth.
Vabysmo has put up a stellar performance against Regeneron’s Eylea, whose sales were under pressure in 2023. However, the approval of a higher dose of Eylea might pose challenges for Vabysmo.
Moreover, competition from biosimilars for established drugs like Avastin, MabThera/Rituxan and Herceptin continues to hurt sales.
Zacks Rank & Stock to Consider
Roche currently carries a Zacks Rank #4 (Sell).
A better-ranked stock in the healthcare industry is Puma Biotechnology, Inc. PBYI, which currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has remained constant at 73 cents. During the same time frame, the consensus estimate for 2024 EPS has remained steady at 69 cents. Over the past year, shares of PBYI have surged 63.1%.
PBYI’s earnings beat estimates in three of the trailing four quarters and missed the same in one, delivering an average surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
