Rocket (RCKT) Surges 40% in 3 Months on Regulatory Updates
Rocket Pharmaceuticals RCKT, a late-stage biotechnology company, has skyrocketed 40.1% in the past three months against the industry’s decline of 11.1%.
Investors have cheered the pipeline progress and positive regulatory updates in this time frame.
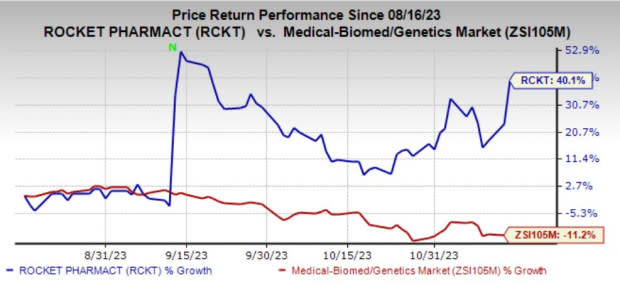
Image Source: Zacks Investment Research
Last month, the FDA accepted the company’s biologics license application (“BLA”) to pipeline candidate RP-L201 (marnetegragene autotemcel), a lentiviral vector-based investigational gene therapy. The BLA was seeking approval of RP-L201 (now Kresladi) for severe leukocyte adhesion deficiency-I (LAD-I), a rare genetic immune disorder that predisposes patients to recurrent and fatal infections and is near-uniformly fatal in children without an allogeneic hematopoietic stem cell transplant.
The regulatory granted Priority Review to the BLA and set a target action date of Mar 31, 2024.
A potential approval of this gene therapy will be a significant boost for this company, which currently has no approved drugs in its portfolio.
The company reported better-than-expected results last week. It reported a loss of 75 cents in the third quarter, which was narrower than the Zacks Consensus Estimate of 82 cents.
Earlier, shares surged in September after the company reached an alignment with the FDA on the mid-stage study design for RP-A501 in Danon Disease.
Danon Disease, a uniformly fatal inherited cardiomyopathy, is caused by mutations in the lysosome-associated membrane protein 2 (LAMP-2) gene.
The company initiated a phase II study of RP-A501 for Danon Disease following alignment with the FDA. The trial is evaluating the safety and efficacy of RP-A501 at a dose level of 6.7 x 1013 GC/kg and includes a pediatric safety run-in (n=2). The co-primary endpoint is composed of LAMP2 protein expression and left ventricular mass index and will be assessed at 12 months for accelerated approval.
A phase I study of RP-A601 for PKP2 arrhythmogenic cardiomyopathy has been initiated.
Rocket has also reached an agreement with the FDA on the study design of the phase II study of RP-L301 for pyruvate kinase deficiency. The company is initiating a 10-patient, single-arm mid-stage study.
In September, the company also completed a public offering of approximately 9.5 million shares of its common stock at $16.00 per share and pre-funded warrants to purchase 3.1 million shares of common stock at $15.99 per warrant. The net proceeds from the offering was $188.9 million.
As of Sep 30, 2023, Rocket had cash, cash equivalents and investments of $437.2 million. Rocket expects that the cash resources will be sufficient to fund its operations through 2025.
Zacks Rank and Other Stocks to Consider
Rocket Pharmaceuticals currently has a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the overall healthcare sector are Dynavax Technologies DVAX and Ligand Pharmaceuticals LGND, each sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Dynavax’s loss per share estimates for 2023 have narrowed from 23 cents to 12 cents for 2023 in the past 30 days. During the same period, earnings estimates for 2024 rose from 3 cents to 18 cents. Shares of DVAX have gained 29.7% year to date.
Earnings estimates for Ligand Pharmaceuticals’ 2023 earnings per share have increased from $4.98 to $5.10 in the past 60 days. During the same period, earnings estimates for 2024 rose from $4.26 to $4.59.
Ligand beat earnings estimates in each of the last four quarters. The company has delivered an earnings surprise of 67.19%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Rocket Pharmaceuticals, Inc. (RCKT) : Free Stock Analysis Report
