Satsuma Pharmaceuticals Announces Topline Results from SUMMIT Phase 3 Trial of STS101 for the Acute Treatment of Migraine


Figure 1
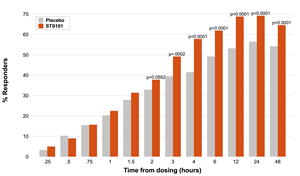

Figure 2


STS101 was not statistically superior to placebo at two hours post-administration on the co-primary endpoints of freedom from pain and most bothersome symptom
STS101 showed superiority (p<0.001) differences versus placebo on freedom from pain and most bothersome symptom at all timepoints after two hours post-administration (3, 4, 6, 12, 24 and 48 hours), as well as on multiple key secondary endpoints, including pain relief at 2 hours post-administration and all timepoints thereafter
STS101 demonstrated a favorable safety and tolerability profile, consistent with clinical trial experience to date
Based on previous interactions with the FDA, Satsuma believes results from the STS101 Phase 1 PK and ASCEND Phase 3 long-term, open-label safety trials will support NDA filing and approval
Company does not plan to invest in commercializing STS101 and will actively explore alternatives to maximize value for shareholders, while minimizing cash expenditures
Estimated cash and equivalents as of October 31, 2022: $59.4 million
Management to host investor call and webcast at 8:30 am EST today
SOUTH SAN FRANCISCO, Calif., Nov. 14, 2022 (GLOBE NEWSWIRE) -- Satsuma Pharmaceuticals, Inc. (Nasdaq: STSA), a clinical-stage biopharmaceutical company developing STS101 (dihydroergotamine (DHE) nasal powder), a novel investigational therapeutic product candidate for the acute treatment of migraine, today reported topline results from the STS101 SUMMIT Phase 3 efficacy trial.
Although topline data showed numerical differences in favor of STS101 5.2 mg versus placebo on the pre-specified co-primary endpoints of freedom from pain and freedom from most bothersome symptom (from among photophobia, phonophobia and nausea) (MBS-free) at two hours post-administration, these differences did not achieve statistical significance (p-value <0.05).
STS101 did, however, demonstrate significant effects on both freedom-from-pain and MBS-free endpoints by three hours post-dose and at all subsequent timepoints at which efficacy was assessed (4, 6, 12, 24 and 48 hours). (See Figures 1 and 2 below.)
Figure 1: % of subjects Pain Free over time (single dose of STS101; no rescue medications)
https://www.globenewswire.com/NewsRoom/AttachmentNg/295eef04-efd1-40b1-8cd3-5b44216aee16
Figure 2: % of subjects free from MBS over time (single dose of STS101; no rescue medications)
https://www.globenewswire.com/NewsRoom/AttachmentNg/e9f52ed8-75b6-472c-bc2b-65eaa0a9706a
In addition, STS101 was statistically superior to placebo on multiple key secondary endpoints considered clinically relevant and recommended for assessment in acute-treatment-of-migraine efficacy trials by the U.S. Food and Drug Administration (FDA) in its current industry guidance document and/or the International Headache Society’s guidelines for controlled trials:1,2
Proportion of subjects with pain relief at 2 hours post-dose (p=0.0017) and at all timepoints thereafter
Proportion of subjects requiring rescue medication within 24 hours and 48 hours post-treatment (p<0.0001 and p=0.0001, respectively)
Proportion of subjects with 24-hour sustained freedom from pain, i.e., the proportion of subjects who reported no headache pain at 2 hours post-dose who remained free from pain at all timepoints through 24 hours (p=0.0479)
Consistent with clinical trial experience to date, STS101 demonstrated a favorable safety and tolerability profile in SUMMIT. The only treatment-emergent adverse event reported by more than 5% of SUMMIT subjects who self-administered STS101 was nasal discomfort, reported by 8.3% of subjects. No treatment-related serious adverse events or cardiovascular events occurred.
“We are surprised and disappointed that STS101 did not demonstrate statistically significant superiority over placebo at two-hours post treatment on the SUMMIT study co-primary endpoints,” stated John Kollins, Satsuma’s President and Chief Executive Officer. “However, based on our interactions to date with the FDA, we believe the results from the STS101 Phase 1 pharmacokinetic and ASCEND Phase 3 open-label, long-term safety trials will support the STS101 NDA filing and marketing approval. A large proportion of people with migraine do not achieve sufficient and sustained relief with current treatments, and we believe that in total, the data from the STS101 clinical program, in which more than 1,600 subjects treated more than 8,500 migraine attacks with more than 10,000 doses of STS101, demonstrate that STS101 has a differentiated profile and may address the unmet needs of many patients.”
In addition, Satsuma announced that it does not plan to invest in commercializing STS101 and will actively explore alternatives to maximize value for shareholders, while minimizing cash expenditures. As of October 31, 2022 the company had estimated cash and equivalents $59.4 million.
Conference Call and Webcast Details
Satsuma management will host a conference call and accompanying webcast for investors at 8:30 am EST this morning. Interested participants may register for the call using the following link: https://conferencingportals.com/event/yMGurUMf
The call can then be accessed by dialing 888-330-2513 (toll free) or 240-789-2726 (toll) and referencing conference ID 49226-446.
The accompanying webcast, which will feature a slide presentation, can be accessed at the following link: https://event.on24.com/wcc/r/4024205/07BAE6AA1EF580725929A36DE3450D67
Participants are asked to dial in approximately ten minutes prior to the start of the call.
The webcast slide presentation is also available in the Events and Presentations page of our website: https://investors.satsumarx.com/events
About Satsuma Pharmaceuticals and STS101
Satsuma Pharmaceuticals is a clinical-stage biopharmaceutical company developing a novel therapeutic product, STS101, for the acute treatment of migraine. STS101 is a unique and proprietary nasal powder formulation of the well-established anti-migraine drug, dihydroergotamine mesylate (DHE), administered via Satsuma’s proprietary nasal delivery device. STS101 is designed to provide significant benefits versus existing acute treatments for migraine, including the combination of quick and convenient self-administration and other clinical advantages, that current DHE liquid nasal spray products and injectable dosage forms lack. Satsuma’s dry powder DHE formulation has demonstrated fast absorption, rapid achievement of high DHE plasma concentrations which Satsuma believes is necessary for robust efficacy, and sustained DHE plasma levels over time with low dose-to-dose variability. STS101 also now incorporates an improved 2nd-generation nasal delivery device designed to provide more consistent nasal dosing, irrespective of user administration technique. DHE has long been recommended in published migraine treatment guidelines as a first-line acute treatment option for migraine and has significant advantages versus other anti-migraine treatments for many patients. However, disadvantages of current DHE liquid nasal spray and injectable products, including invasive and burdensome administration and/or sub-optimal clinical performance, have limited the widespread use of DHE. Featuring an easy-to-carry and easy-to-use dosage form, STS101 is designed to overcome these shortcomings and provide patients an improved therapeutic solution for acutely treating migraines that consistently delivers robust clinical performance.
Satsuma is headquartered in South San Francisco, California with operations in both California and Research Triangle Park, North Carolina. For further information, please visit www.satsumarx.com.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements concerning the business, operations and financial performance and condition of Satsuma Pharmaceuticals, Inc. (the “Company”), as well as the Company’s plans, objectives and expectations for its business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about the Company’s expectations regarding the potential safety and efficacy of STS101, the potential results of the ASCEND and SUMMIT trials, the timing of data readouts for ongoing clinical trials, the anticipated timing for a potential STS101 NDA submission, the potential for STS101 to be an important and differentiated acute treatment option, and the expected cash runway of the Company. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, filed with the Securities and Exchange Commission, as well as other documents that may be filed by the Company from time to time. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of STS101; the results of preclinical and clinical studies may not be predictive of future results; and the risk that the COVID-19 worldwide pandemic may negatively impact the Company’s business, operations, clinical trials or ability to raise capital. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
INVESTOR AND CORPORATE CONTACTS:
Corey Davis, PhD
LifeSci Advisors, LLC
212-915-2577
cdavis@lifesciadvisors.com
Tom O’Neil, Chief Financial Officer
Satsuma Pharmaceuticals, Inc.
tom@satsumarx.com
1 FDA Guidance, Migraine: Developing Drugs for Acute Treatment, February 2018
2 Diener et al., Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth Edition, Cephalalgia, 2019

