Solid Biosciences (SLDB) Stock Up as FDA Clears DMD Drug IND
Solid Biosciences Inc. SLDB announced that the FDA has cleared its investigational new drug (IND) application to begin clinical studies on its novel gene therapy candidate, SGT-003, for the treatment of Duchenne Muscular Dystrophy (DMD).
SGT-003 is being developed using Solid Biosciences’ proprietary and rationally designed capsid (AAV-SLB101).
The IND clearance was based on encouraging data from the company’s pre-clinical study of SGT-003. In the pre-clinical study, SGT-003 demonstrated increased biodistribution to cardiac and skeletal muscle, including the diaphragm, compared with AAV9 in nonhuman primates.
Furthermore, the company believes that the pre-clinical data of the candidate suggest increased transgene expression and an improved safety profile compared with first-generation microdystrophin gene therapies.
The company’s stock surged 27.9% in the last trading session due to the FDA’s nod to begin clinical studies on SGT-003 in DMD. Year to date, shares of Solid Biosciences plunged 49.3% compared with the industry’s 22.5% fall.
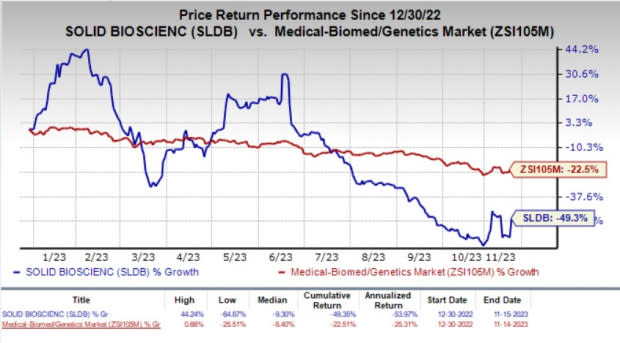
Image Source: Zacks Investment Research
Following the IND clearance of SGT-003, the company is gearing up to receive IRB approval at the study sites and expects to commence patient screening shortly thereafter. The planned phase I/II study of the candidate will evaluate the safety and tolerability of SGT-003 in pediatric patients with DMD at a dose of 1E14vg/kg.
In the early-stage study of the candidate, SGT-003 will be administered as a one-time intravenous infusion to patients in two cohorts with a minimum of three patients each. Solid Biosciences is also keeping a provision for potential cohort expansion in the study if deemed necessary.
Per SLDB, cohort 1 of the phase I/II study will evaluate SGT-003 in patients aged 4 to < 6 years of age with DMD. The long-term safety and efficacy of the candidate will be evaluated for a total of five years following treatment.
DMD is a progressive and degenerative disorder that leads to weakness and wasting away of the body’s muscles.
Solid Biosciences Inc. Price and Consensus
Solid Biosciences Inc. price-consensus-chart | Solid Biosciences Inc. Quote
Currently, Sarepta Therapeutics SRPT markets the only approved one-shot gene therapy drug in the United States. The FDA granted accelerated approval to Sarepta’s Elevidys (delandistrogene moxeparvovec or SRP-9001), an adeno-associated virus-based gene therapy, to treat ambulatory pediatric patients aged between four and five years with DMD in June.
Elevidys has been developed by Sarepta in collaboration with Roche RHHBY. Both Sarepta and Roche entered into a licensing agreement in 2019 to jointly develop and commercialize Elevidys. Per the agreement, RHHBY has exclusive rights to launch and commercialize the gene therapy in ex-U.S. markets.
The FDA had, however, restricted the use of Elevidys in DMD to patients aged between four and five years, in consultation with the Sarepta, due to concerns regarding the ambiguity revolving around the benefit that Elevidys will provide to DMD patients. The agency’s non-age-restricted expansion to Elevidys was contingent upon the success of the phase III EMBARK study of Elevidys in DMD.
In October 2023, Sarepta announced that the confirmatory phase III EMBARK study missed its primary endpoint. However, data from the study supports the conclusion that treatment with Elevidys modifies the course of DMD indication. Management claims to have shared these results with the FDA, who have confirmed that they are open to such label expansion if supported by a review of the data and that they intend to proceed rapidly with consideration of the submission. Sarepta intends to submit a regulatory filing with the agency in 2023.
To satisfy regulatory requirements for Elevidys’ approval outside the United States, SRPT also initiated the phase III ENVISION study to evaluate the safety and efficacy of gene therapy in non-ambulatory and ambulatory DMD patients.
PTC Therapeutics PTCT also currently markets Emflaza (deflazacort) in the United States for the treatment of DMD in patients aged five years and older. Emflaza is a non-gene therapy product. It was developed by Marathon Therapeutics and received FDA approval in 2017 as the first drug in the United States for DMD, regardless of genetic mutation.
In 2017, PTC Therapeutics acquired Emflaza from Marathon for an upfront payment of $140 million. PTC remains liable to make tiered royalty payments to Marathon based on annual net sales of Emflaza beginning in 2018.
PTCT’s Emflaza is a corticosteroid that demonstrates anti-inflammatory and immunosuppressant effects.
Zacks Rank
Solid Biosciences currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
Solid Biosciences Inc. (SLDB) : Free Stock Analysis Report
