Sotherly Hotels' (SOHO) Q2 Operating Trend Reflects Solid Demand
Shares of Viking Therapeutics VKTX have surged 63.7% in the year so far against the industry’s 12.0% fall.
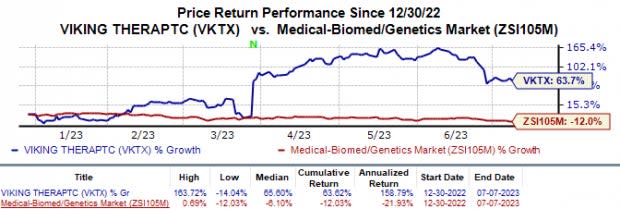
Image Source: Zacks Investment Research
The upside can be attributed to the successful development of its pipeline candidates targeting various metabolic and endocrine disorders.
In March, Viking Therapeutics reported encouraging data from the recently completed early-stage clinical study evaluating its dual GIP/GLP-1-RA drug VK2735 administered subcutaneously across various metabolic disorders. Data from the study demonstrated up to 6% placebo-adjusted mean weight reduction (7.8% from baseline), following 28 days of treatment with VK2735. Management also believes that the tolerability data from this study suggest that higher doses of VK2735 may be achieved with longer titration windows. Based on these results, Viking intends to start a phase II study evaluating VK2735 to treat patients with obesity by mid-2023.
Based on the results, Viking Therapeutics initiated an early-stage extension study to evaluate the oral formulation of VK2735 with highly-differentiated dosing options in healthy adults. The initiation is likely in response to the ongoing obesity drug wave wherein several large-cap pharma companies like Eli Lilly LLY, Novartis and Pfizer are evaluating oral formulations for treating obesity.
Earlier this year, several pharma companies reported positive data from multiple studies evaluating their obesity drugs. Eli Lilly is running multiple comprehensive clinical development programs evaluating different drugs for treating obesity in patients, with or without diabetes, which can be administered orally or by injections. Last month, Lilly reported new data from multiple mid-stage studies, evaluating different obesity drug that achieved a mean weight reduction of up to 24%. LLY also completed the regulatory submission seeking label expansion for its dual GIP/GLP-1-RA drug tirzepatide to treat obesity or overweight in adults, with a final decision expected before this year’s end. Shares of Viking Therapeutics suffered a negative impact following Lilly’s announcements.
In May, Viking Therapeutics reported positive data from an ongoing phase IIb study (VOYAGE) evaluating VK2809 in patients with biopsy-confirmed non-alcoholic steatohepatitis (“NASH”). The VOYAGE study achieved its primary endpoint of statistically significant reduction in liver fat content in NASH patients following 12 weeks of treatment with VK2809. In fact, 85% of patients who received VK2809, experienced at least a 30% relative reduction in liver fat content. Management plans to report 52-week treatment data from this study in first-half 2024.
NASH is a progressive form of non-alcoholic fatty liver disease, characterized by excessive fat buildup in the liver, accompanied by inflammation and fibrosis, which may progress to cirrhosis, liver failure, cancer and death.
While the NASH market holds potential with no approved therapies yet, it is challenging as several companies like Madrigal Pharmaceuticals MDGL and Akero Therapeutics AKRO are trying to develop a successful treatment for the same.
Madrigal Pharmaceuticals is developing its lead candidate resmetirom for treating NASH and liver fibrosis. Last December, Madrigal reported positive top-line data from the pivotal phase III MAESTRO-NASH study evaluating its resmetirom for treating NASH and liver fibrosis. The study achieved its primary endpoints and potentially clinically meaningful effects compared with placebo. Based on this data, Madrigal initiated a rolling new drug application (NDA) submission last month seeking accelerated approval for resmetirom to treat NASH with liver fibrosis.
Akero Therapeutics is evaluating its lead candidate efruxifermin (EFX) for treating NASH. Akero is on track to report 36-week treatment data from the phase IIb SYMMETRY main study, evaluating EFX in adult patients with cirrhotic NASH in fourth-quarter 2023. Akero Therapeutics is also on track to initiate two phase III studies as part of the late-stage SYNCHRONY program in second-half 2023 to evaluate EFX in NASH.
Currently, Viking Therapeutics does not have a single marketed drug in its portfolio. A successful development of its pipeline candidates will help boost the company’s growth prospects. Other than VK2809 and VK2735, Viking is also evaluating VK0214, a novel oral TRβ agonist, in an ongoing phase Ib study, for X-linked adrenoleukodystrophy (X-ALD), a rare neurogenerative disease for which there are currently no pharmacologic treatment options. Initial results from the study are expected before 2023-end.
Viking Therapeutics, Inc. Price
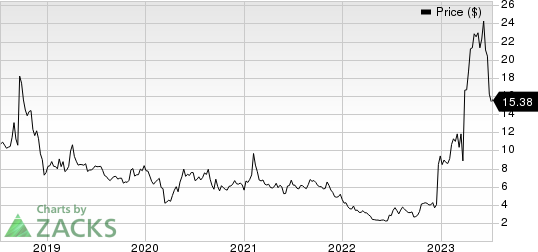
Viking Therapeutics, Inc. price | Viking Therapeutics, Inc. Quote
Zacks Rank
Viking Therapeutics currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Iron Mountain Incorporated (IRM) : Free Stock Analysis Report
Ventas, Inc. (VTR) : Free Stock Analysis Report
W.P. Carey Inc. (WPC) : Free Stock Analysis Report
Sotherly Hotels Inc. (SOHO) : Free Stock Analysis Report
