Spectral Medical Announces Positive Results of EUPHAS-2 Clinical Trial Evaluating PMX in Critically Ill Endotoxemic Septic Shock Patients


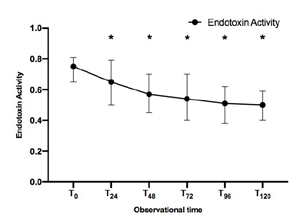
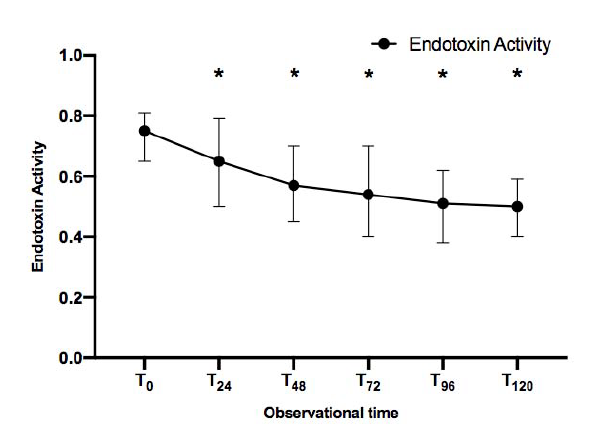
Trend of Endotoxin Activity over time in all patients included in the study

Reports 28-day mortality of just 36% for patients undergoing PMX hemoadsorption versus
75% predicted by widely accepted SAPS II mortality estimation tool
Results featured in premier peer-reviewed journal highlighting the benefits of PMX
TORONTO, April 18, 2023 (GLOBE NEWSWIRE) -- Spectral Medical Inc. (“Spectral” or the “Company”) (TSX: EDT), a late-stage theranostic company advancing therapeutic options for sepsis and septic shock, today announced positive results in EUPHAS-2, an observational study in Italy using EAA-guided PMX hemoadsorption as a treatment for patients with endotoxemic septic shock. The results were featured in Artificial Organs, a premier, peer-reviewed journal publishing the latest in research, development, translation, and clinical application of organ replacement therapies, and organ machine perfusion preservation.
The study analysis included 50 patients with septic shock and endotoxin activity (EAT0) ≥ 0.6, who received Polymyxin-B hemoadsorption (PMX) therapy. The primary outcome of the study was the endotoxin activity (EA) score using Spectral’s Endotoxin Activity Assay (EAA™), and Sequential Organ Failure Assessment (SOFA) score progression over 120 hours.
Among patients in the study, septic shock was mainly caused by 27 abdominal (54%) and 17 pulmonary (34%) infections. Over the course of 120 hours, a decrease in EA was observed (p<0.001), as well as an improvement in both SOFA score and hemodynamics (p<0.001).
In addition, the Simplified Acute Physiology Score II (SAPS II), a widely accepted severity score and mortality estimation tool utilized by the medical community, was 67.5 [52.8-82.3], which predicted a 28-day mortality rate of 75%. In contrast, the 28-day mortality among patients who received PMX was 18 patients (36%). In patients whose high EA resolved within 48 hours, progression to kidney failure was lower compared with patients with EAT48≥0.6.
Dr. John Kellum, Chief Medical Officer of Spectral Medical, stated, “We were pleased to see that this cohort of patients with high EAA, 0.75 (0.65-0.81), and high severity of illness (APACHE II 26, SAPS II 67.5) achieved a hospital survival rate of 64% with PMX therapy. The results of the EUPHAS-2 study confirm our results from the US subgroup enrolled in the EUPHRATES trial who also had significant organ failure (multiple organ dysfunction score >9) and EAA between 0.6 and 0.89. In this intent-to-treat cohort of 179 patients, 28-day mortality was 36.7%. Furthermore, as seen in EUPHAS-2 in patients with decreasing EAA, recovery from septic shock was faster. We are currently prospectively studying PMX therapy compared to standard of care in patients with very similar disease severity (and EAA levels) to those enrolled in the EUPHAS 2 study.”
Chris Seto, CEO of Spectral Medical, commented, “We are excited to report these encouraging results, along with publication of the data in a long-standing, peer-reviewed journal. The patients in this observational study had similar profile to patients being recruited in our current confirmatory Tigris trial and reinforce our prior findings, including preliminary data from the Tigris trial. Notably, in critically ill patients with septic shock and a high level of endotoxemia, reductions in endotoxin activity with PMX therapy was associated with improved organ function based on SOFA scores, better hemodynamics and fewer cases of kidney failure requiring renal replacement therapy. Importantly, our preliminary Tigris mortality data continues to exceed our expectations, and based on this latest data, we remain confident in our ability to achieve a successful trial outcome. We also continue to advance enrollment in the Tigris trial and believe that the continued onboarding of new Tigris sites will allow us to more rapidly reach our 150-patient target, bringing us closer to FDA submission and potential FDA approval.”
About Spectral
Spectral is a Phase 3 company seeking U.S. FDA approval for its unique product for the treatment of patients with septic shock, Toraymyxin™ (“PMX”). PMX is a therapeutic hemoperfusion device that removes endotoxin, which can cause sepsis, from the bloodstream and is guided by the Company’s Endotoxin Activity Assay (EAA™), the only FDA cleared diagnostic for the risk of developing sepsis.
PMX is approved for therapeutic use in Japan and Europe, and has been used safely and effectively on more than 340,000 patients to date. In March 2009, Spectral obtained the exclusive development and commercial rights in the U.S. for PMX, and in November 2010, signed an exclusive distribution agreement for this product in Canada. In July 2022, the U.S. FDA granted Breakthrough Device Designation for PMX for the treatment of endotoxemic septic shock. Approximately 330,000 patients are diagnosed with septic shock in North America each year.
Spectral is listed on the Toronto Stock Exchange under the symbol EDT. For more information please visit www.spectraldx.com.
Forward-looking statement
Information in this news release that is not current or historical factual information may constitute forward-looking information within the meaning of securities laws. Implicit in this information, particularly in respect of the future outlook of Spectral and anticipated events or results, are assumptions based on beliefs of Spectral's senior management as well as information currently available to it. While these assumptions were considered reasonable by Spectral at the time of preparation, they may prove to be incorrect. Readers are cautioned that actual results are subject to a number of risks and uncertainties, including the availability of funds and resources to pursue R&D projects, the successful and timely completion of clinical studies, the ability of Spectral to take advantage of business opportunities in the biomedical industry, the granting of necessary approvals by regulatory authorities as well as general economic, market and business conditions, and could differ materially from what is currently expected.
The TSX has not reviewed and does not accept responsibility for the adequacy or accuracy of this statement.
For further information, please contact:
Ali Mahdavi | David Waldman/Natalya Rudman | Blair McInnis |
|
Capital Markets & Investor Relations | US Investor Relations | CFO |
|
Spinnaker Capital Markets Inc. | Crescendo Communications, LLC | Spectral Medical Inc. |
|
416-962-3300 | 212-671-1020 | 416-626-3233 |
|
|
A chart accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/fab2e783-ebf3-452a-b92a-85f2a8f25479

