Surmodics (SRDX) Rallies on FDA Approval for SurVeil DCB
Surmodics, Inc. SRDX announced that the FDA has approved its new medical device — SurVeil drug-coated balloon (DCB). The SurVeil DCB will now be available in the United States to treat percutaneous transluminal angioplasty in patients with peripheral artery disease (PAD). The device is threaded into a blood vessel, which is then inflated to open blocked or narrowed areas in the same.
The approval is based on data from a 24-month pivotal clinical study that evaluated SurVeil DCB for non-inferiority to Medtronic’s MDT IN.PACT Admiral DCB. Data from the study demonstrated the sustained durability of SurVeil DCB for safety and effectiveness that was non-inferior to MDT’s IN.PACT Admiral DCB.
Almost 81.8% of patients treated with SurVeil DCB attained freedom from device and procedure-related death as well as major target limb amputation compared to 83.2% of those treated with IN.PACT Admiral DCB. Less than 15% of patients needed repeat revascularization procedures for both DCBs.
It’s important to note that while both SurVeil DCB and IN.PACT Admiral DCB use paclitaxel drug coatings, the latter has a 75% higher drug load of paclitaxel compared with the former.
Medtronic’s IN.PACT Admiral DCB is being used for treating percutaneous transluminal angioplasty for several years. The device is also a market leader in its category in certain regions, especially Europe. A non-inferior study result for SurVeil DCB, with a substantially lower drug dose, should help in overcoming competition from IN.PACT Admiral DCB.
Price Performance
Shares of Surmodics rallied 16.1% on Jun 20, following the FDA approval for SurVeil DCB. Surmodics stock has lost 18.2% in the past year compared with the industry’s 20.4% decline. The S&P 500 Index has risen 17% in the same time frame.
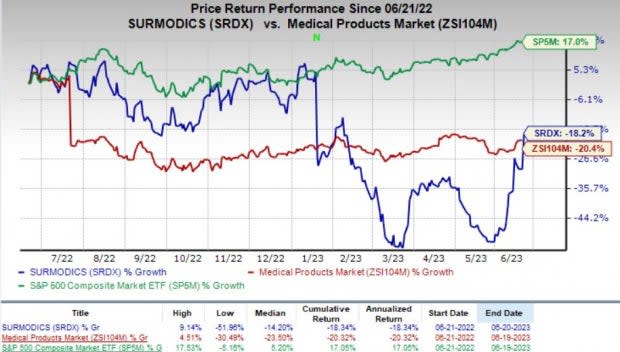
Image Source: Zacks Investment Research
FDA Review History
SRDX completed submission of a pre-market approval (PMA) application to the FDA for SurVeil DCB in 2021. In January, the company announced a negative regulatory outcome. It stated that the FDA rejected the PMA application, requesting additional biocompatibility and labeling. The adverse regulatory outcome had an unfavorable impact on the company’s business outlook, leading to several layoffs.
Although Surmodics adopted several cost-cutting measures following this setback, it was in discussion with the FDA for regulatory pathway for a potential approval for SurVeil DCB. In March, the company received formal feedback from the FDA related to its proposed approach to submit a PMA application.
Based on the regulatory authority’s feedback, Surmodics submitted an amended PMA application during the third quarter of fiscal 2023. The approval came earlier than the scheduled time, which was in the fourth quarter of fiscal 2023 (September end).
Please note that SurVeil DCB is already approved in Europe for similar indication since 2020.
Commercialization Plan
Surmodics has a deal in place with Abbott Laboratories ABT for commercialization of SurVeil DCB. Per the terms of the agreement, Abbott has exclusive worldwide commercialization rights for SRDX’s DCB.
SRDX is responsible for manufacturing and supply of the product to Abbott. It will also record these sales as product revenues. The company will receive a share of profit on third-party sales made by ABT.
Per the deal, Surmodics is eligible to receive a milestone payment of $27 million from Abbott, following the FDA approval for SurVeil DCB. SRDX expects revenues in the range of $24.0-$24.5 million related to the milestone payment in the third quarter.
Industry Prospects
Per a report by InsightAce Analytics, the DCB market was valued at $600.3 million in 2019 and is anticipated to grow at a CAGR of 16.7% during 2020-2028. According to the same report, application of DCBs for treating PAD patients is likely to almost double by 2030 from the 2021 level.
Factors like the prevalence of peripheral and coronary artery diseases, and growth in demand for minimally invasive procedures are likely to drive the market.
Given the market potential, the latest regulatory clearance raises optimism for Surmodics.
Notable Development
Earlier this month, Surmodics announced its receipt of the FDA’s 510(k) clearance for its Pounce LP (Low Profile) Thrombectomy System. The company expects to initiate limited market evaluation (LME) for the Pounce LP Thrombectomy System by the end of the first quarter of fiscal 2024.
SRDX is planning the commercialization of SurVeil DCB, following the completion of the LME. The Pounce Thrombectomy System was intended for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in 3.5-6 mm thick vessels.
In April, Surmodics reported fiscal second-quarter 2023 results, wherein it experienced a solid uptick in the overall top line. The company registered robust revenues from its Medical Device segment. It also recorded growth in Product sales, and Research, development and other revenues.During the quarter, Surmodics saw strong contributions from sales of its Pounce and Sublime products, indicating their continued solid demand.
The same month, the company announced the enrollment of the first patient in PROWL, the Pounce Thrombectomy System Retrospective Registry. PROWL is an open-label, retrospective, multi-center U.S. registry of the Surmodics Pounce system for the non-surgical removal of emboli and thrombi in the peripheral arterial vasculature.
Also, in April, Surmodics announced the first usage of the company’s Sublime radial access microcatheter, which is currently in limited market evaluation. The full suite of Sublime microcatheters will be launched in fiscal 2024.
Surmodics, Inc. Price
Surmodics, Inc. price | Surmodics, Inc. Quote
Zacks Rank & Another Stock to Consider
Currently, Surmodics carries a Zacks Rank #2 (Buy).
Another top-ranked stock in the broader medical space is Haemonetics HAE, sporting a Zacks Rank #1 (Strong Buy) at present. Haemonetics has an estimated growth rate of 5.1% for fiscal 2024. HAE’s earnings surpassed estimates in each of the trailing four quarters, delivering an average surprise of 27.3%. You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ shares have risen 37.9% in the past year against the industry’s 20.4% decline.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Abbott Laboratories (ABT) : Free Stock Analysis Report
Medtronic PLC (MDT) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
Surmodics, Inc. (SRDX) : Free Stock Analysis Report
