Teleflex's (TFX) Arrow EZ-IO Needle Receives FDA Approval
Teleflex Corporation TFX recently announced that the FDA has granted 510(k) clearance to e Arrow EZ-IO Needle — the first and only intraosseous (IO) needle — for MR Conditional labeling. The innovative diamond tip of the EZ-IO Needle, a crucial part of the Arrow EZ-IO Intraosseous Vascular Access System, is made for rapid, exact and steady insertion.
The recent development will bolster Teleflex’s Vascular Access segment.
More on the News
The EZ-IO System can prove beneficial when intravenous access is difficult to obtain due to an emergency, an urgent situation or a medical necessity. The new labeling enables the practitioner to continue treating patients who need MRI scans without interrupting the vascular access site's operation.
To enhance patient care, Clinical and Medical Affairs is dedicated to promoting the increased usage of Teleflex medical devices. Now, there is a second vascular access choice for patients who need an urgent or emergent MRI.
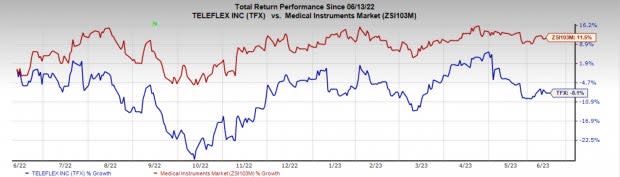
Image Source: Zacks Investment Research
Teleflex continues developing technologies for physicians who want to offer enhanced and consistent care in urgent situations. The company is optimistic about the Arrow EZ-IO Needle receiving MR Conditional clearance and is eager to explore new avenues to provide patients and healthcare professionals with excellent treatment solutions.
Industry Prospects
Per a report by Grand View Research, the global vascular access devices market size was valued at $1.77 billion in 2022 and is anticipated to witness a CAGR of 5% by 2030. Patient demographics — the aging global population, the rising cancer prevalence and the widespread metabolic, respiratory and cardiovascular disorders — are expected to fuel market growth.
Progress Within Vascular Access Segment
Following the $976-million acquisition of Vascular Solutions, there has been accelerated growth within Teleflex’ vascular and interventional access product portfolios.
During the reported quarter, the company introduced two new products intended to improve PICC insertion procedures and lower the risk of problems. First, the business introduced the next-generation Arrow VPS Rhythm DLX device, which uses a patient's cardiac electrical activity to offer real-time PICC catheter tip location information. Using the DLX eliminates the need for confirmatory x-rays that shortens the time it takes to start therapy and lowers the cost.
Teleflex introduced a new Arrow PICC that already has preloaded NaviCurve Stylet. A flexible tip and anatomical curb on the NaviCurve Stylet allow it to self-orient to the patient's anatomy for effective PICC insertion into the superior vena cava.
Within interventional business, the company relaunched Langston catheter that progressed through the first quarter with product availability in the United States, Canada Australia and New Zealand. The Langston catheter continues to build value for the company’s customers, enhance its engagement with clinicians in TAVR and demonstrates its relevance in the structural heart space. Teleflex expects further product launches in the interventional business over the coming years, including complex catheters in the structural part market.
In June 2023, Teleflex received the FDA clearance for Wattson Temporary Pacing Guidewire. The company will feature Wattson at TVT — The Structural Heart Summit between Jun 7-10 in Phoenix. The latest development will expand the Structural Heart Portfolio, with the first commercially-available bipolar temporary pacing guidewire designed specifically for use during transcatheter aortic valve replacement (TAVR) and balloon aortic valvuloplasty (BAV).
Price Performance
Shares of the company have dropped 11.2% in the past year compared with the industry’s fall of 4.5%.
Zacks Rank and Key Picks
Teleflex carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Merit Medical Systems, Inc. MMSI and Boston Scientific Corporation BSX.
Hologic, carrying a Zacks Rank #2 (Buy) at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 13.4% compared with the industry’s 11..5% rise in the past year.
Merit Medical, carrying a Zacks Rank #2, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 53.4% compared with the industry’s 16.9% rise over the past year.
Boston Scientific, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11.5%. BSX’s earnings surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 1.9%.
Boston Scientific has gained 40.9% against the industry’s 24.5% decline in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Teleflex Incorporated (TFX) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report
