TG Therapeutics (TGTX) Gets FDA Nod for Multiple Sclerosis Drug
TG Therapeutics, Inc. TGTX announced that the FDA has approved its anti-CD20 monoclonal antibody, Briumvi (ublituximab-xiiy), for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.
Following the FDA nod, Briumvi became the first and only anti-CD20 monoclonal antibody to be approved for patients with RMS that can be administered in a one-hour infusion twice a year after the starting dose.
The company plans to launch Briumvi in the United States in the first quarter of 2023.
Shares of TG Therapeutics were up 8.2% on Wednesday following the announcement of the news. However, the stock has plunged 56.3% in the past year compared with the industry’s decline of 44.9%.
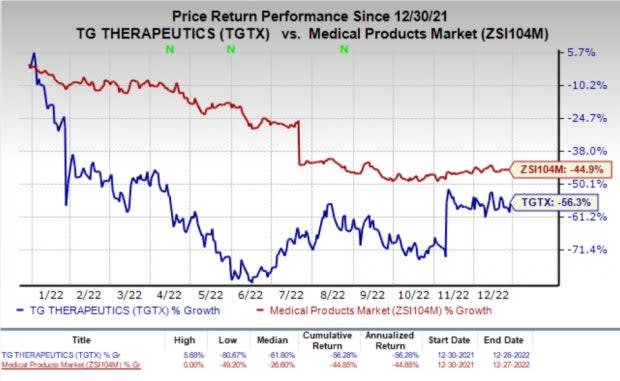
Image Source: Zacks Investment Research
The FDA approval for Briumvi was based on data from the phase III ULTIMATE I & II studies, which evaluated Briumvi against Sanofi’s SNY Aubagio (teriflunomide) in patients with RMS.
Both ULTIMATE I and ULTIMATE II studies achieved their primary endpoint, where treatment with Briumvi demonstrated superiority over Sanofi’s Aubagio by showing a statistically significant reduction in the annualized relapse rate.
We remind investors that in May 2022, the FDA extended the review period of TGTX’s biologics licensing applications for Briumvi as a potential treatment for patients with RMS.
Following this three-month extension, the FDA set a new PDUFA action date of Dec 28, 2022. A decision from the regulatory body was previously expected on Sep 28, 2022.
Aubagio is a blockbuster drug acquired by Sanofi upon buying Genzyme Corporation. The drug is approved by the FDA for treating RMS. During the first nine months of 2022, SNY’s Aubagio sales declined 4.2%, hurt by competitive pressure and price in the United States.
Importantly, the FDA approval of Briumvi is likely to offer a new option to patients with RMS, which can be administered in a one-hour infusion every six months after receiving the first dose of Briumvi.
Successful commercialization of Briumvi will boost TG Therapeutics’ growth prospects in the days ahead.
Zacks Rank & Stocks to Consider
TG Therapeutics currently carries a Zacks Rank #3 (Hold). Top-ranked stocks in the biotech sector are Syndax Pharmaceuticals, Inc. SNDX and Celularity Inc. CELU, both carrying a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 5.9% for 2022 and 14.5% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Loss per share estimates for Celularity have narrowed 57.1% for 2022 and 7.7% for 2023 in the past 60 days.
Earnings of Celularity surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. CELU witnessed an earnings surprise of 51.01% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
TG Therapeutics, Inc. (TGTX) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Celularity, Inc. (CELU) : Free Stock Analysis Report
