Thermo Fisher's (TMO) PPD Business Backs BARDA's Clinical Trial
Thermo Fisher Scientific’s TMO PPD clinical research business has been recently selected by the Biomedical Advanced Research and Development Authority (BARDA) to implement the first BARDA-supported Phase II platform clinical trial. The trial will investigate multiple therapeutic options for the treatment of acute respiratory distress syndrome (ARDS), a life-threatening lung condition without any approved or licensed therapeutics available presently.
BARDA is part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services. The latest opportunity allows Thermo Fisher to lend its expertise and innovative solutions for the development of effective treatments for ARDS, both in the United States and worldwide.
Relevance of the PPD Clinical Research Business
Thermo Fisher’s PPD clinical research business has more than three decades of experience delivering clinical research services and has conducted studies on a global scale across all trial phases and a broad array of therapeutic areas. During the past five years, it has conducted more than 175 respiratory studies involving more than 40,000 patients at nearly 10,000 research sites around the world.
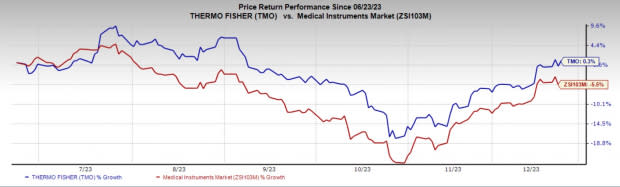
Image Source: Zacks Investment Research
The business has also supported more than 450 government-funded projects, including full-service clinical trials, statistical and data coordinating centers, clinical site monitoring centers, regulatory management centers and clinical research operations and management support programs for BARDA, multiple National Institutes of Health institutes and centers, the Centers for Disease Control and Prevention and the U.S. Department of Defense.
News in Detail
ARDS can stem from multiple causes, including severe pneumonia and sepsis due to bacterial and viral infections such as influenza and SARS-CoV-2, that lead to high rates of death among hospitalized patients. A critical and unaddressed medical need currently exists to better understand the clinical and biological features of ARDS, to devise an optimal treatment for patients suffering from this grave condition.
The primary objective of the BARDA-funded, hypothesis-generating study is to gather comprehensive clinical and biomarker data on the ARDS patient population to better inform future clinical studies and contribute to the development of targeted therapies for ARDS. Under this contract, the PPD clinical research business will implement a large Phase II clinical trial over three years. The randomized, double-blind, placebo-controlled, multicenter Phase II platform trial will evaluate the safety and efficacy of the three host-directed therapeutics at up to 60 U.S. sites, enrolling 600 hospitalized adult patients with ARDS. The drug candidates in the study will be announced in early 2024.
The PPD clinical research business will support the clinical trial with its deep capabilities and expertise in end-to-end platform clinical trials, seamless biomarker testing, critical care, drug depot and laboratory services.
Industry Prospects
Per a Research report, the global ARDS market was valued at $129.66 billion in 2022 and is expected to witness a CAGR of 10.1% through the 2023-2030 period.
Recent Developments
Earlier this week, the company announced the launch of CorEvidence, a proprietary cloud-based data lake platform optimizing pharmacovigilance case processing and safety data management processes. The new platform enhances CorEvitas clinical research registries offered by Thermo Fisher’s PPD clinical research business.
The CorEvitas acquisition was completed in August 2023 and complements Thermo Fisher’s renowned clinical research business with real-world evidence solutions. The high-growth market segment is an increasingly important area for the company’s pharmaceutical and biotechnology customers, which will help to enhance decision-making as well as the time and cost of drug development.
Price Performance
In the past six months, TMO shares have increased 0.3% against the industry’s 5.5% fall.
Zacks Rank and Key Picks
Thermo Fisher currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the broader medical space are Haemonetics HAE, Insulet PODD and DexCom DXCM. Haemonetics and DexCom each presently carry a Zacks Rank #2 (Buy), and Insulet sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ stock has risen 7.9% in the past year. Earnings estimates for Haemonetics have increased from $3.86 per share to $3.89 per share in 2023 and $4.11 per share to $4.15 per share in 2024 in the past 30 days.
HAE’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 16.1%. In the last reported quarter, it posted an earnings surprise of 5.3%.
Estimates for Insulet’s 2023 earnings per share have moved up from $1.90 to $1.91 in the past 30 days. Shares of the company have dropped 25% in the past year compared with the industry’s decline of 2.6%.
PODD’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 105.1%. In the last reported quarter, it delivered an average earnings surprise of 77.4%.
Estimates for DexCom’s 2023 earnings per share have increased from $1.43 to $1.44 in the past 30 days. Shares of the company have increased 9% in the past year compared with the industry’s growth of 2.1%.
DXCM’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 36.4%. In the last reported quarter, it delivered an average earnings surprise of 47.1%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Thermo Fisher Scientific Inc. (TMO) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
DexCom, Inc. (DXCM) : Free Stock Analysis Report
Insulet Corporation (PODD) : Free Stock Analysis Report
