Ultragenyx (RARE) Doses First Patients in Bone Disease Studies
Ultragenyx Pharmaceutical Inc. RARE reported dosing the first group of patients in both its late-stage studies evaluating setrusumab (UX143) in young adults and pediatric patients with Osteogenesis Imperfecta (OI) sub-types I, III and IV. Last month, Ultragenyx and its partner, Mereo BioPharma MREO, announced positive data from the mid-stage portion of the study of setrusumab for patients with OI.
In the phase II dose-selection portion of phase II/III, the Orbit study’s data revealed that the 20 mg/kg dose of setrusumab rapidly induces bone production and bone mineral density in patients affected by OI. These endpoints are important markers of bone strength as well as early indications of improved bone health.
Based on positive results from the study’s phase II portion of the study, the phase III portion of the pivotal phase II/III Orbit study was initiated to evaluate setrusumab’s efficacy compared with placebo on the annualized clinical fracture rate in patients aged 5 to <26 years. Additionally, Ultragenyx also initiated another phase III study, Cosmic, to evaluate setrusumab compared with intravenous bisphosphonate (IV-BP) therapy on the annualized total fracture rate in patients aged 2 to <5 years.
Ultragenyx’s shares have lost 1.8% year to date compared with the industry’s 9.9% decline.
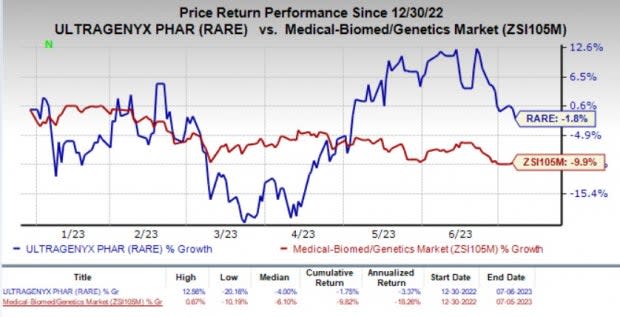
Image Source: Zacks Investment Research
Setrusumab, a fully human monoclonal antibody, is an inhibitor of the sclerostin protein, which is responsible for hampering the maturation and activity of bone-forming cells.
We would like to remind the investors that Ultragenyx entered into a licensing and collaboration agreement with Mereo in December 2020 to co-develop setrusumab. Per the terms of the agreement, Ultragenyx was tasked with leading the future global development of setrusumab in both pediatric and adult patients with OI. Under this deal, Mereo received an upfront payment of $50 million from Ultragenyx. Mereo also remains eligible to receive up to $254.0 million upon achieving certain regulatory and sales-based milestones.
In return, Mereo granted Ultragenyx an exclusive license to develop and commercialize setrusumab in the United States, Turkey and the rest of the world. However, Mereo retained its commercial rights in the European Economic Area, the U.K. and Switzerland, also known as the Mereo Territory. Furthermore, Ultragenyx is responsible for bearing global development costs as well as making tiered royalty payments to Mereo based on net sales in its territory. Mereo is also liable to pay Ultrageyx a fixed royalty on net sales in the Mereo Territory.
The pivotal phase III portion of the phase II/III Orbit study will enroll approximately 195 patients and divide them into two cohorts in a ratio of 2:1, receiving either a 20 mg/kg dose of setrusumab or placebo. The primary efficacy endpoint of the study is the annualized clinical fracture rate. Per Ultragenyx, all patients will transition to an extension period and receive open-label setrusumab after completing the primary analysis in the phase III portion of the study.
On the other hand, the phase III cosmic study of setrusumab in pediatric patients with OI is set to enroll approximately 65 patients. The primary efficacy endpoint of this study is the reduction in total fracture rate, including morphometric vertebral fractures, upon treatment with setrusumab in comparison to treatment with IV-BP.
OI is a group of genetic disorders that impact bone metabolism. It is characterized by increased bone fragility and a high risk of fractures. The condition affects approximately 60,000 people in the developed world. Currently, there are no approved treatments for this debilitating condition, representing a highly unmet medical need.
Ultragenyx Pharmaceutical Inc. Price and Consensus
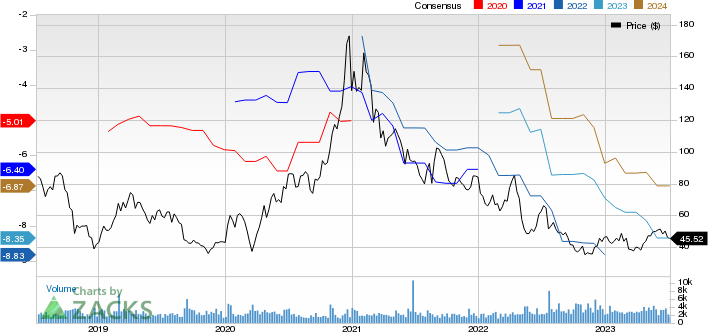
Ultragenyx Pharmaceutical Inc. price-consensus-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank and Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
Some top-ranked stocks in the biotech sector are 89BIO ETNB and Akero Therapeutics AKRO, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for 89BIO’s 2023 loss per share has narrowed from $2.37 to $1.92. During the same period, the estimate for AKRO’s 2024 loss per share widened from $2.33 to $2.49. Year-to-date, shares of ETNB have gained 45.5%.
ETNB beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 11.27%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.92 to $2.80. During the same period, the estimate for AKRO’s 2024 loss per share narrowed from $3.31 to $3.27. Year-to-date, shares of AKRO have lost 17.8%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
MEREO BIOPHARMA (MREO) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
89BIO (ETNB) : Free Stock Analysis Report
