Ultragenyx (RARE) Q4 Loss Narrower Than Expected, Revenues Beat
Ultragenyx Pharmaceutical Inc. RARE incurred a loss of $1.52 per share in fourth-quarter 2023, narrower than the Zacks Consensus Estimate of a loss of $1.65. The company reported a loss of $2.16 per share in the year-ago quarter.
Ultragenyx’s total revenues amounted to $127 million in the reported quarter, up 23% year over year. The top line beat the Zacks Consensus Estimate of $122 million. The uptick was mainly driven by increased Crysvita and Dojolvi sales.
The company markets four drugs, namely Crysvita, Mepsevii, Dojolvi and Evkeeza. Crysvita is approved for treating X-linked hypophosphatemia, an inherited disorder and tumor-induced osteomalacia, an ultra-rare disease. Mepsevii is approved to treat Mucopolysaccharidosis VII, also known as Sly syndrome. Dojolvi was approved in June 2020 for all forms of long-chain fatty acid oxidation disorders.
In January 2022, Ultragenyx announced a license and collaboration agreement with Regeneron Pharmaceuticals REGN. Per the deal, RARE has obtained the rights to develop, commercialize and distribute Evkeeza (evinacumab) outside the United States. The regions include the European Economic Area. The collaboration with Regeneron for Evkeeza gives Ultragenyx a fourth-approved product that adds to the top line. However, REGN will continue to solely commercialize Evkeeza in the United States.
Shares of RARE have gained 4.4% in the past year against the industry’s 10.5% decline.
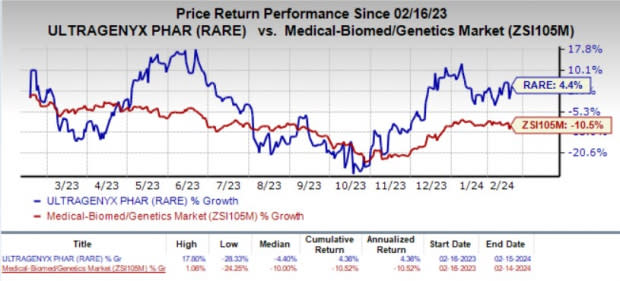
Image Source: Zacks Investment Research
Quarter in Detail
Crysvita’s total revenues were $94 million, up 17% year over year, driven by increased demand for approved indications. Crysvita’s net product revenues in fourth-quarter 2023 were primarily driven by sales of the drug in the Latin America region and Turkey, which were $18 million, representing 139% year-over-year growth. Ultragenyx sold its Crysvita rights in the European territory to Royalty Pharma in December 2019. In April 2023, RARE entered into a licensing agreement withKyowa Kirin, transitioning commercialization responsibilities for Crysvita in North America to its partner.
Mepsevii product revenues were $7.9 million in the fourth quarter, up 64% from the year-ago quarter. Dojolvi product revenues were $23.3 million, up 42% from the year-ago quarter, driven by strong new patient demand. Evkeeza recorded sales of $2.1 million in the reported quarter.
Operating expenses of $249 million in the reported quarter were relatively flat year over year. Operating expenses for the reported quarter include research and development expenses of $161 million (down 6%) and selling, general and administrative expenses of $77 million (up 5%).
Cash, cash equivalents and marketable debt securities amounted to $777.1 million as of Dec 31, 2023, compared with $524.2 million as of Sep 30, 2023.
Full-Year 2023 Results
Revenues in 2023 came in at $434 million, up 20% from $363 million in 2022. The net loss per share came in at $8.25, which is narrower than a loss of $10.12 reported in 2022.
2024 Guidance
Ultragenyx expects total revenues in 2024 between $500 million and $530 million. Crysvita revenues are expected in the range of $375-$400 million (including all regions where Ultragenyx will recognize revenues, comprising product sales in Latin America and Turkey, royalties in Europe, which have been ongoing, and the royalties in North America, which began in April 2023). Dojolvi revenues are expected between $75 million and $80 million.
Net cash used in Operations is expected to be less than $400 million in 2024.
Ultragenyx Pharmaceutical Inc. Price, Consensus and EPS Surprise
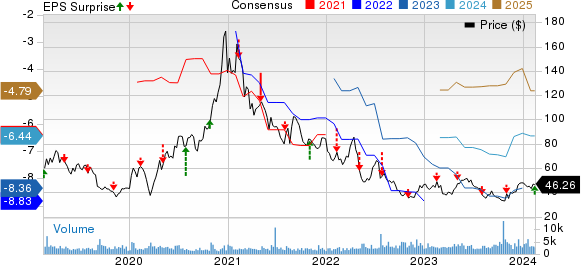
Ultragenyx Pharmaceutical Inc. price-consensus-eps-surprise-chart | Ultragenyx Pharmaceutical Inc. Quote
Pipeline Updates
Ultragenyx and its partner, Mereo BioPharma MREO, are jointly developing UX143 (setrusumab) monoclonal antibody for pediatric and young adult patients with osteogenesis imperfecta in two late-stage studies, Orbit and Cosmic. The phase III portion of the Orbit study is expected to complete enrollment by the end of the first quarter of 2024. Additional longer-term phase II safety and efficacy data from the Orbit study are expected in the second half of 2024.
The phase III Cosmic study is evaluating the effect of setrusumab compared with intravenous bisphosphonate therapy on the annualized total fracture rate in patients aged 2 to <5 years. The Cosmic study is anticipated to complete enrollment in the first half of 2024.
Per the ongoing collaboration deal, Mereo received an upfront payment of $50 million from Ultragenyx, granting the latter an exclusive license to setrusumab. MREO also remains eligible to receive up to $254 million upon achieving certain regulatory and sales-based milestones. Furthermore, RARE is responsible for bearing global development costs as well as making tiered royalty payments to Mereo based on net sales in its territory. Mereo is also liable to pay Ultrageyx a fixed royalty on net sales in the Mereo Territory.
Ultragenyx is also developing GTX-102 for treating patients with Angelman syndrome (AS) in an early to mid-stage study. Enrollment in the expansion cohorts of the phase I/II study was completed in December 2023. The expansion cohorts will evaluate many of the same safety, pharmacokinetic and efficacy measures as the previously enrolled dose-escalation/extension cohorts, plus some new evaluations.
Top-line results from the AS study are expected in the first half of 2024 from 20 expansion cohort patients on therapy for at least 170 days.
The company expects to achieve several other milestones in 2024 for other candidates in its pipeline undergoing clinical development.
Zacks Rank & a Stock to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the same industry is Puma Biotechnology, Inc. PBYI, sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has remained constant at 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has remained constant at 69 cents. Over the past year, shares of PBYI have gained 67.4%.
PBYI beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
MEREO BIOPHARMA (MREO) : Free Stock Analysis Report
