Ultragenyx's (RARE) Preliminary 2023 Revenues Disappoint
Ultragenyx Pharmaceutical RARE reported preliminary total revenues (unaudited) of $430-$435 million for 2023, up 18-20% from 2022. The Zacks Consensus Estimate is pegged at $436.22 million.
The company markets four drugs, such as Crysvita, Mepsevii, Dojolvi and Evkeeza. Crysvita is approved for treating X-linked hypophosphatemia, an inherited disorder and tumor-induced osteomalacia, an ultra-rare disease. Mepsevii is approved to treat Mucopolysaccharidosis VII, also known as Sly syndrome. Dojolvi was approved in June 2020 for all forms of long-chain fatty acid oxidation disorders.
Per the press release, preliminary revenues from the sale of Crysvita in 2023 were $325-$330 million, up 16-18% year over year and Dojolvi net product sales were $70-$71 million, up 25-27% from the 2022 figure.
RARE reported cash, cash equivalents and available-for-sale investments of approximately $776 million as of Dec 31, 2023, according to management’s initial analysis of operations. Per the company, cash uses in 2023 included the completion of construction of its gene therapy manufacturing facility.
Ultragenyx has also issued its 2024 revenue guidance. Total revenues in 2024 are expected to be in the range of $500-$530 million in 2024, up 16-22% from 2023. Crysvita and Dojolvi revenues are expected to be in the range of $375-$400 million (up 15-21%) and $75-$80 million (up 7-13%), respectively, in 2024. The company also expects its net cash use in 2024 to be less than $400 million.
Over the past year, shares of Ultragenyx have gained 17.2% against the industry’s 11.4% decline.
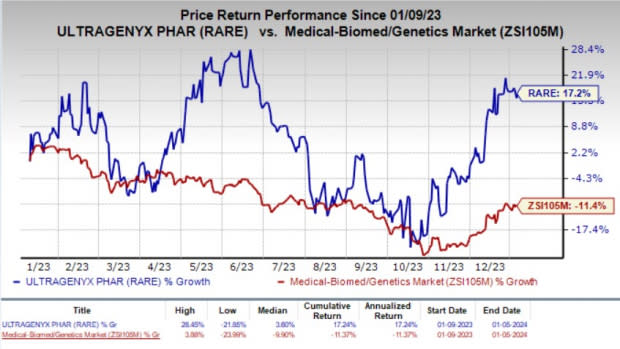
Image Source: Zacks Investment Research
In the same press release, the company has also provided updates regarding its pipeline programs, along with certain upcoming milestones in 2024.
Ultragenyx and its partner, Mereo BioPharma MREO, are currently dosing patients in the two late-stage studies, ORBIT and COSMIC, evaluating UX143 (setrusumab), a monoclonal antibody, in pediatric and young adult patients with osteogenesis imperfecta (OI).
Per the company’s ongoing collaboration agreement with Mereo to co-develop UX143, RARE was tasked with leading the future global development of UX143 in both pediatric and adult patients with OI. Under this deal, Mereo received an upfront payment of $50 million from Ultragenyx. MREO also remains eligible to receive up to $254 million upon achieving certain regulatory and sales-based milestones.
RARE expects to complete enrollment in the phase III portion of the ORBIT study around the end of the first quarter of 2024. The company also expects to report additional longer-term data from the phase II portion of the ORBIT study in 2024.
The phase III COSMIC study evaluates the effect of setrusumab compared with intravenous bisphosphonate therapy on the annualized total fracture rate in patients aged 2 to <5 years. The company expects to enroll a total of approximately 65 patients in the study by the first half of 2024.
Ultragenyx recently announced that it has completed enrollment in the expansion cohorts of the phase I/II study of GTX-102, an antisense oligonucleotide, for Angelman Syndrome. The company expects to report results from at least 20 expansion cohort patients on therapy for at least six months in the first half of 2024.
The company has completed dosing four out of five patients in the third and final dose escalation cohort in its pivotal phase I/II/III Cyprus2+ study evaluating UX701 gene therapy to treat Wilson Disease. The fifth patient is reportedly scheduled to be dosed soon. Stage 1 of the study evaluates the safety and efficacy of UX701, and a dose will be selected for further evaluation in Stage 2, which is the pivotal portion of the study.
Ultragenyx expects data readout from Stage 1 in the first half of 2024, followed by dose selection and initiation of Stage 2 in the second half of 2024.
The phase III study of DTX401 gene therapy for glycogen storage disease Type Ia is also currently ongoing. The study has completed enrollment and expects to report top-line results in the first half of 2024. The primary endpoint of the late-stage study is the reduction in oral glucose replacement with cornstarch while maintaining glucose control.
Another phase III study evaluating DTX301 gene therapy for ornithine transcarbamylase deficiency is currently dosing patients. Enrollment in the study is expected to be completed in the first half of 2024. The primary endpoints of the late-stage study are response as measured by the removal of ammonia-scavenger medications and protein-restricted diet and change in 24-hour ammonia levels.
Ultragenyx Pharmaceutical Inc. Price and Consensus
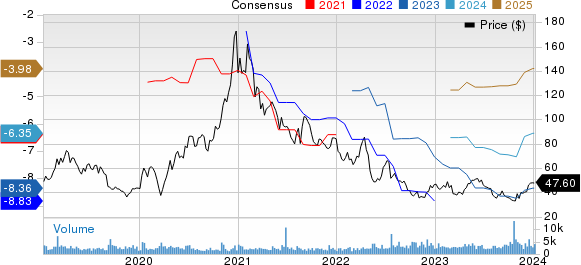
Ultragenyx Pharmaceutical Inc. price-consensus-chart | Ultragenyx Pharmaceutical Inc. Quote
Zacks Rank & Other Stocks to Consider
Ultragenyx currently carries a Zacks Rank #2 (Buy).
Some other top-ranked drug/biotech stocks worth mentioning are Puma Biotechnology, Inc. PBYI and ADMA Biologics ADMA. While PBYI sports a Zacks Rank #1 (Strong Buy), ADMA carries a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has increased from 72 cents to 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 64 cents to 69 cents. Over the past year, shares of PBYI have gained 1.7%.
PBYI’s earnings beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 3 cents to 2 cents. The consensus estimate for ADMA Biologics’ 2024 EPS is pegged at 18 cents. Over the past year, shares of ADMA have gained 25.4%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 63.57%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
MEREO BIOPHARMA (MREO) : Free Stock Analysis Report
