Vanda (VNDA) Falls on FDA's CRL to Hetlioz sNDA for Insomnia
Shares of Vanda Pharmaceuticals Inc. VNDA were down 5.6% on Mar 6 after the company announced that the FDA issued a complete response letter (CRL) to a supplemental new drug application (sNDA) for its marketed product, Hetlioz (tasimelteon), on Mar 4.
The sBLA sought approval for Hetlioz for the treatment of insomnia, which is characterized by difficulties with sleep initiation.
The FDA notified the company that it had identified deficiencies that precluded discussion of labeling and post-marketing requirements/commitments related to the Hetlioz sNDA early last month. No deficiencies were disclosed by the FDA in the notification.
The regulatory body issued the CRL stating that it cannot approve the sNDA in its current form.
Shares of Vanda have plunged 36.9% in the past year compared with the industry’s decline of 3.7%.
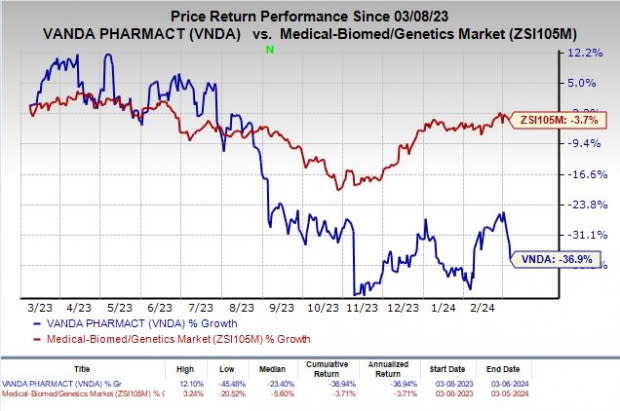
Image Source: Zacks Investment Research
The company submitted the sNDA for Hetlioz in insomnia in May 2023. A decision from the FDA was expected on Mar 04, 2024.
Hetlioz is currently approved for the treatment of non-24-hour sleep-wake disorder and nighttime sleep disturbances in Smith-Magenis syndrome.
As Hetlioz is facing the risk of generic launches, the company is working to expand its label. However, the latest CRL to the sNDA for Hetlioz is likely to delay the approval and the expected launch of the drug for a new indication.
VNDA is also looking to pursue the FDA’s approval for Hetlioz in jet lag disorder.
Another approved product in Vanda’s commercial portfolio is Fanapt, which is approved for the treatment of schizophrenia.
A sNDA seeking approval for Fanapt for treating adult patients with bipolar I disorder is also under review, with a decision from the FDA expected on Apr 2, 2024.
Zacks Rank & Other Stocks to Consider
Vanda currently sports a Zacks Rank #1 (Strong Buy).
Some other top-ranked stocks in the healthcare sector are Indivior PLC INDV, ADMA Biologics, Inc. ADMA and ANI Pharmaceuticals, Inc. ANIP, each carrying a Zacks Rank #1 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Indivior’s 2024 earnings per share have improved from $1.83 to $1.95. In the past year, shares of INDV have risen 4.2%.
Indivior’s earnings beat estimates in each of the trailing three quarters. INDV delivered an average earnings surprise of 48.06%.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have improved from 18 cents to 30 cents. In the past year, shares of ADMA have increased 79.5%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met the same once. ADMA delivered an average earnings surprise of 85.00%.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have improved from $4.06 to $4.22. In the past year, shares of ANIP have risen 52.6%.
Earnings of ANI Pharmaceuticals beat estimates in each of the trailing four quarters. ANIP delivered a four-quarter average earnings surprise of 109.60%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vanda Pharmaceuticals Inc. (VNDA) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Indivior PLC (INDV) : Free Stock Analysis Report
