Vertex (VRTX), CRSP's Casgevy Gets EU Nod for Two Blood Disorders
Vertex Pharmaceuticals VRTX and partner CRISPR Therapeutics CRSP announced that the European Commission has granted conditional marketing approval to their one-shot gene therapy Casgevy for treating two debilitating blood disorders, sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT).
Casgevy is approved for patients 12 years and older with severe SCD characterized by recurrent vaso-occlusive crises (VOCs) or TDT, for whom hematopoietic stem cell (HSC) transplantation is appropriate and a human leukocyte antigen matched related HSC donor is not available.
With the approval of this CRISPR/Cas9 gene-edited therapy, approximately 8,000 patients 12 years of age and older with SCD or TDT in Europe become potentially eligible for treatment. Casgevy is the first gene therapy approved for SCD and TDT in Europe.
In the United States, the FDA approved Casgevy for the SCD indication in December 2023 and for the TDT indication in January 2024. Casgevy (exa-cel) secured its first-ever approval/authorization in the United Kingdom in November 2023.
Casgevy is the first gene therapy developed with the Nobel prize-winning CRISPR technology to be approved anywhere in the world. This technology can selectively delete, modify or correct a disease-causing abnormality in a specific DNA segment. It is also the first marketed product in CRISPR Therapeutics’ portfolio.
Vertex and CRISPR have set a price of $2.2 million for the one-time treatment in the United States.
In the past year, shares of Vertex and CRISPR have risen 38.5% and 43.0%, respectively, against the industry’s decline of 13.0%.
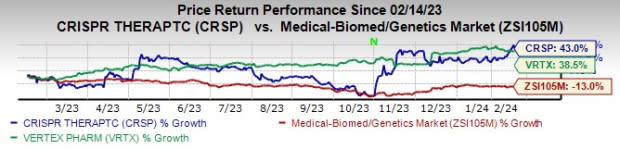
Image Source: Zacks Investment Research
Both SCD and TDT indications have a significant unmet medical need. Casgevy has demonstrated the potential to alleviate blood transfusion requirements for TDT patients as well as reduce painful and debilitating sickle crises for SCD patients. If successfully commercialized, the therapy can reap huge profits for Vertex and CRISPR Therapeutics. Vertex and CRISPR Therapeutics are also conducting two phase III studies evaluating the gene therapy in pediatric patients with TDT and SCD.
bluebird bio BLUE also markets a gene therapy Zyntelgo for TDT. We remind investors that bluebird’s Zyntelgo was approved by the FDA in 2022 as the first gene therapy for TDT.
Apart from Zyntelgo, Vertex/CRISPR also faces stiff competition from bluebird in the SCD space. Alongside Casgevy’s approval in SCD in January, the FDA also approved bluebird’s Lyfgenia gene therapy for the SCD indication.
Some other companies are also using the CRISPR/Cas9 gene editing technology to address various ailments. One such company is Editas Medicine EDIT, which is developing its lead pipeline candidate EDIT-301 that employs CRISPR gene editing to treat SCD and TDT indications. EDIT-301 is in early-stage development. Beam Therapeutics is developing BEAM-101 for the SCD indication in early-stage studies.
Zacks Rank
Vertex and CRISPR have a Zacks Rank #3 (Hold) each. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
