Vertex's (VRTX) Q4 Earnings and Sales Surpass Estimates
Vertex Pharmaceuticals Incorporated VRTX reported fourth-quarter 2023 adjusted earnings per share of $4.20, which beat the Zacks Consensus Estimate of $4.10. Earnings increased 12% year over year. Strong cystic fibrosis (“CF”) product revenues led to higher earnings in the reported quarter.
Revenues of $2.52 billion surpassed the Zacks Consensus Estimate of $2.49 billion. Revenues solely comprised CF product revenues. Total product revenues rose 9.3% year over year, primarily driven by higher sales of Trikafta/Kaftrio (marketed as Kaftrio in Europe) in the U.S. and ex-U.S. markets.
Quarter in Detail
The company currently markets four CF products — Trikafta/Kaftrio, Symdeko (marketed as Symkevi in Europe), Orkambi and Kalydeco.
CF product sales rose 8% year over year in the United States to $1.57 billion, while sales outside the United States increased 12% to $943 million.
Trikafta generated sales worth $2.33 billion, up 15.4% year over year. The upside was driven by the continued robust performance of Trikafta in the United States, fueled by label expansions to younger age groups (two to five years old). In the ex-U.S. markets, the drug continues to witness strong uptake with recently achieved reimbursements and expanded use in young age groups. Trikafta sales beat the Zacks Consensus Estimate and our model estimate of $2.29 billion and $2.28 billion, respectively.
Sales from other CF products, namely Symdeko/Symkevi, Kalydeco and Orkambi, were down 34.4% year over year to $184.4 million. Sales of these drugs were hurt by patients switching to Trikafta.
Full-Year Results
For 2023, Vertex generated revenues of $9.87 billion, reflecting 11% growth year over year.
For the same period, the company reported adjusted earnings of $15.23 per share, up 2.4% year over year.
Shares of Vertex have rallied 41.3% in the past year against the industry’s decline of 15%.
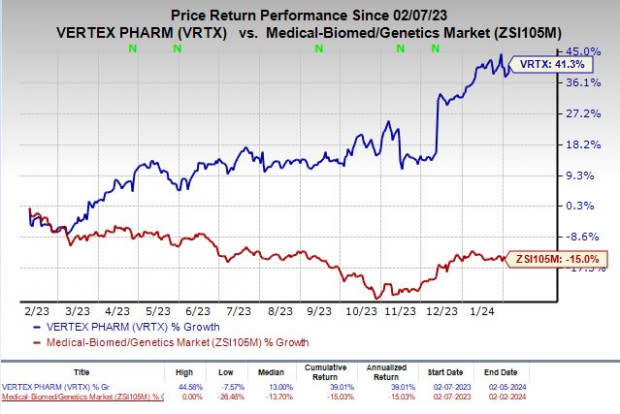
Image Source: Zacks Investment Research
Costs Rise
Adjusted R&D expenses rose 12.1% from the year-ago quarter’s levels to $698.8 million due to the expansion of the company’s mid- and late-stage pipeline, especially the pivotal studies for VX-548 in acute pain.
Adjusted selling, general and administrative (SG&A) expenses increased 26.6% to $285.6 million in the reported quarter due to expenses for CF launches and commercial launch activities for Casgevy (exa-cel), which has been developed in partnership with CRISPR Therapeutics CRSP.
During the reported quarter, Vertex recorded acquired in-process research and development (IPR&D) costs of $17.8 million compared with $22.6 million in the year-ago quarter.
2024 Guidance
The company expects total product sales in the range of $10.55-$10.75 billion for 2024, including sales from newly approved gene therapy, Casgevy. The Zacks Consensus Estimate for total revenues is pegged at $10.58 billion for 2024.
Combined adjusted R&D, acquired IPR&D and SG&A expense guidance for 2024 is in the band of $4.3-$4.4 billion. The adjusted tax rate is expected to be in the range of 20-21%.
Posts Positive Data on Triple Combination of Vanzacaftor
In a separate press release, Vertex announced positive data from the pivotal SKYLINE 102 and SKYLINE 103 studies, evaluating the efficacy and safety of the vanzacaftor/tezacaftor/deutivacaftor combo, in comparison with Trikafta in patients with CF aged 12 years and older. The company also announced positive data from the pivotal RIDGELINE study of the same vanzacaftor triple combination therapy in children with CF aged six to 11 years.
All the three studies met the primary endpoint and all key secondary endpoints. Based on these data, VRTX plans to submit an NDA for the vanza triple for treating people with CF aged six years and older to the FDA using a priority review voucher by mid-2024.
This new once-a-day combination medicine has the potential for enhanced patient benefit than Trikafta and can potentially treat CF patients who have discontinued Trikafta or other Vertex CF medicines.
Pipeline Update
In December 2023, the FDA approved Vertex and CRISPR Therapeutics’ Casgevy for treating sickle cell disease (“SCD”) in patients aged 12 years and older.
Last month, Vertex and CRISPR Therapeutics announced that the FDA expanded the Casgevy label to treat transfusion-dependent beta thalassemia (“TDT”) in patients aged 12 years and older.
The commercial launch of Casgevy is currently underway in the United States, United Kingdom, Saudi Arabia and Bahrain. The company expects the first commercial patients to start treatment with Casgevy in the upcoming weeks.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (“EMA”) had adopted a positive opinion, recommending conditional approval of Casgevy to treat SCD and TDT in December 2023.
VRTX and CRSP are awaiting a potential approval for Casgevy in the European Union.
In January 2024, Vertex announced positive data from three late-stage studies on its investigational orally-administered non-opioid pain medicine, VX-548, to treat moderate-to-severe acute pain.
The company evaluated VX-548 in two pivotal phase III acute pain studies — one following bunionectomy surgery and the other following abdominoplasty surgery. The studies achieved their primary endpoint — treatment with VX-548 showed a significant reduction in pain intensity across a 48-hour period compared with placebo.
Vertex also evaluated VX-548 for up to 14 days in a single-arm late-stage study across a broad range of other surgical and non-surgical acute pain conditions. Data from the study showed that around 83% of study participants rated the drug as “good, very good, or excellent” in treating pain.
Based on the above data, Vertex plans to submit a new drug application (“NDA”) with the FDA for VX-548 across a broad label in moderate-to-severe acute pain by mid-2024.
Vertex Pharmaceuticals Incorporated Price, Consensus and EPS Surprise
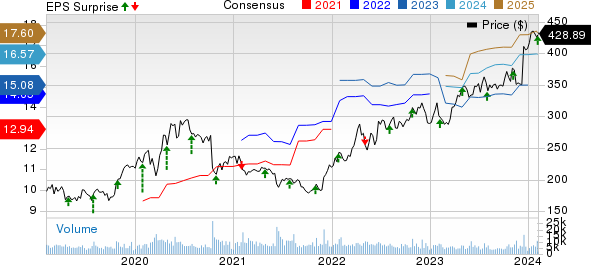
Vertex Pharmaceuticals Incorporated price-consensus-eps-surprise-chart | Vertex Pharmaceuticals Incorporated Quote
Zacks Rank & Stocks to Consider
Vertex currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Kodiak Sciences Inc. KOD and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Kodiak Sciences’ 2024 loss per share have narrowed from $3.70 to $3.66. In the past year, shares of KOD have plunged 45.8%.
Kodiak Sciences beat estimates in two of the last four quarters and missed the same on the remaining two occasions. KOD delivered a four-quarter average earnings surprise of 4.33%.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 64 cents to 69 cents. In the past year, shares of PBYI have risen 2.1%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
Kodiak Sciences Inc. (KOD) : Free Stock Analysis Report
