What's in Store for Regeneron Pharmaceuticals (REGN) in 2023?
Regeneron Pharmaceuticals REGN outperformed the biotech industry in 2022 and is all set for a steady performance in 2023 as well on the back of a solidly diversified portfolio and encouraging pipeline progress.
Regeneron’s lead drug, Eylea, approved for various ophthalmology indications, has been a consistent performer and has maintained momentum with sustained label expansions.
Regeneron had earlier announced that the two studies evaluating aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration were successful. The company is evaluating a higher dose of aflibercept with less frequent injections in the targeted population. The successful label expansion of the drug in this indication will further propel sales and boost the top line.
Regeneron is jointly developing aflibercept 8 mg with Bayer AG BAYRY. While REGN records net product sales of Eylea in the United States, Bayer records net product sales of the drug outside the United States. Regeneron records its share of profits/losses in connection with sales of Eylea outside the United States.
Regeneron’s top line is also boosted by its share of profits/losses in connection with global sales of Dupixent. Partner Sanofi SNY records global net product sales of Dupixent. Solid sales of Dupixent (approved for use in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis) have boosted the sales of the drug. Dupixent was recently approved by the European Commission (EC) to treat adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy.
Regeneron is also looking to diversify in the lucrative oncology space with Libtayo (cemiplimab-rwlc), indicated in certain patients with advanced basal cell carcinoma, advanced cutaneous squamous cell carcinoma and advanced non-small cell lung cancer (NSCLC).
The European Commission recently approved the label expansion of Libtayo as a monotherapy for the treatment of adult patients with recurrent or metastatic cervical cancer and disease progression on or after platinum-based chemotherapy. It was also approved in Japan for the same purpose.
Regeneron has approximately 35 product candidates in clinical development, including approved drugs being evaluated for additional indications. The FDA has accepted for Priority Review the supplemental biologics license application (sBLA) for Evkeeza (evinacumab-dgnb) as an adjunct to other lipid-lowering therapies to treat children aged 5 to 11 years with homozygous familial hypercholesterolemia (HoFH). The FDA has set a target action date of Mar 30, 2023.
Regeneron’s shares have gained 17% in the past year against the industry’s decline of 19.1%.
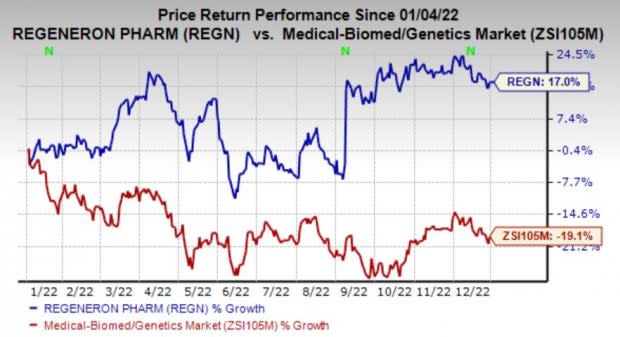
Image Source: Zacks Investment Research
However, stiff competition from the potential entry of generics and new treatment options weighs on Eylea. While Libtayo holds promise, the competition is stiff in this oncology space with many formidable drugs.
The contribution from REGEN-COV, a cocktail of two monoclonal antibodies — casirivimab and imdevimab) — for COVID-19, has seen a massive decline in 2022 from 2021, thereby pulling down year-over-year growth.
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
